268887
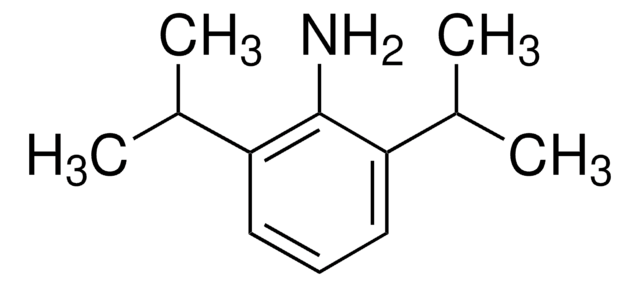
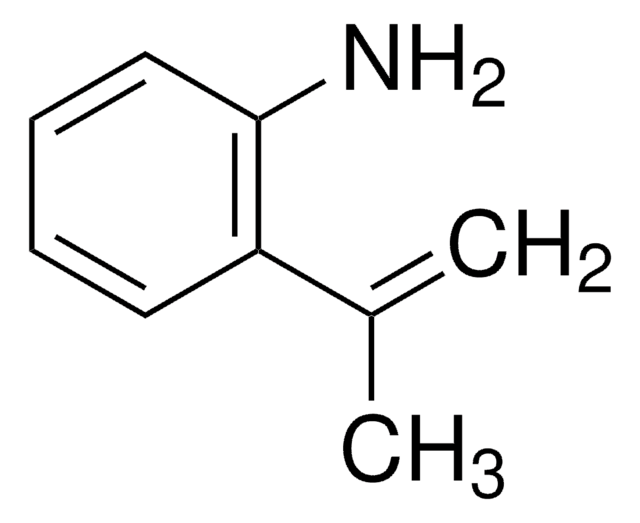
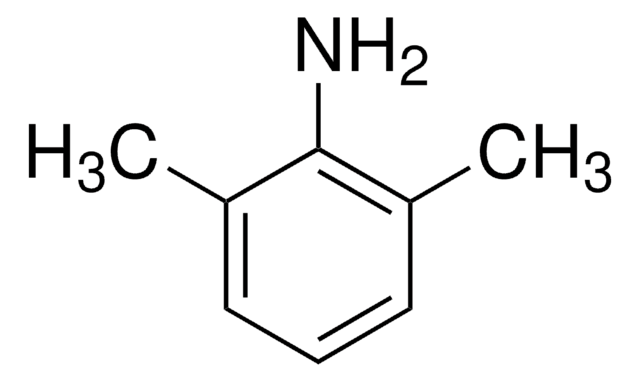
2-Isopropylaniline
97%
Synonym(s):
o-Aminocumene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2CHC6H4NH2
CAS Number:
Molecular Weight:
135.21
Beilstein:
636283
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
0.05 mmHg ( 20 °C)
Assay
97%
form
liquid
refractive index
n20/D 1.548 (lit.)
bp
112-113 °C/18 mmHg (lit.)
density
0.955 g/mL at 25 °C (lit.)
SMILES string
CC(C)c1ccccc1N
InChI
1S/C9H13N/c1-7(2)8-5-3-4-6-9(8)10/h3-7H,10H2,1-2H3
InChI key
YKOLZVXSPGIIBJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The reactions of 1,1′-bis(hydroxymethyl)ferrocene with 2-isopropylaniline, in the presence of a catalyst [RuCl2(PPh3)3], was studied.
Application
2-Isopropylaniline was used in the synthesis of a colorless amine-coordinated zinc complex.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Further Investigation on Preparation, Structure and Electrochemical Properties of N-Alkyl-and N-Aryl-2-aza-[3]-ferrocenophanes.
Sakano T, et al.
Bulletin of the Chemical Society of Japan, 74(11), 2059-2065 (2001)
Junbin Ji et al.
Applied microbiology and biotechnology, 103(15), 6333-6344 (2019-05-24)
The residues of aniline and its derivatives are serious environment pollutants. Aniline dioxygenase (AD) derived from aerobic bacteria catalyzes the conversion of aniline to catechol, which has potential use in the bioremediation of aromatic amines and biorefining process. AD contains
Dongzhen Li et al.
Organic & biomolecular chemistry, 8(8), 1816-1820 (2010-05-08)
Zn(OTf)(2) acts as an excellent catalyst precursor for addition of various amine N-H bonds to carbodiimides under an atmosphere of air, offering a convenient synthesis of substituted guanidines with high functional-group tolerance. A Zn-N amido species is shown to act
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service