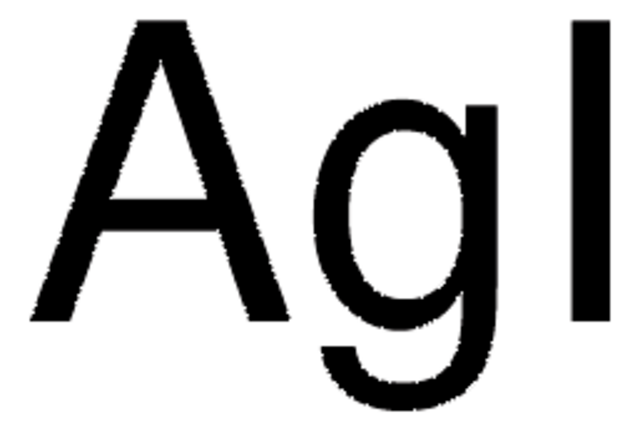All Photos(2)
About This Item
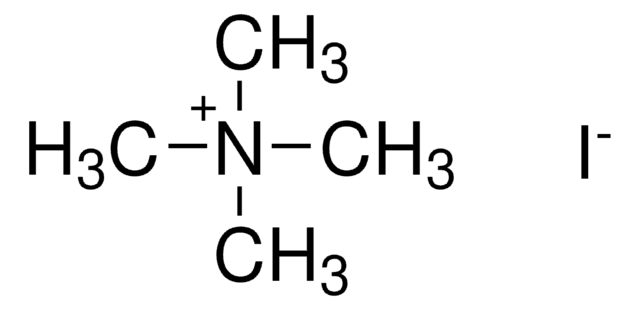
Empirical Formula (Hill Notation):
AgI
CAS Number:
Molecular Weight:
234.77
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Assay:
99.999% trace metals basis
form:
powder and chunks
Recommended Products
Quality Level
Assay
99.999% trace metals basis
form
powder and chunks
impurities
≤20.0 ppm Trace Metal Analysis
density
5.68 g/mL at 25 °C (lit.)
SMILES string
[Ag]
InChI
1S/Ag.HI/h;1H/q+1;/p-1
InChI key
MSFPLIAKTHOCQP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vicente Bitrián et al.
The Journal of chemical physics, 130(23), 234504-234504 (2009-06-25)
The fluctuation-dissipation theorem for the static dielectric response function of systems of ions with inducible point dipoles is derived. It is shown that the static longitudinal dielectric function is determined by spatial correlations of both charge and dipole-moment density fluctuations.
Xunsi Wang et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 86, 586-589 (2011-12-06)
A serious of tellurium based chalcogenide glass were prepared and investigated. As it being transparent nearly up to 25 μm and strong anti-hydrability, it becomes an optimized material for far-infrared application. Here, AgI was incorporated into the glasses acting as
Formation, structure, and polymorphism of novel lowest-dimensional AgI nanoaggregates by encapsulation in carbon nanotubes.
Matteo Baldoni et al.
Small (Weinheim an der Bergstrasse, Germany), 3(10), 1730-1734 (2007-09-13)
Norbert Muntean et al.
The journal of physical chemistry. A, 116(25), 6630-6642 (2012-05-05)
A new type of iodide selective electrode prepared by dipping a silver wire into molten silver iodide is reported. The electrode was calibrated for silver and iodide ions and the measured electromotive force for various Ag(+) and I(-) concentrations was
George N Khairallah et al.
Dalton transactions (Cambridge, England : 2003), (29)(29), 3149-3157 (2007-07-20)
The gas phase ion-molecule reactions of silver cluster cations (Ag(n)(+)) and silver hydride cluster cations (Ag(m)H(+)) with 2-iodoethanol have been examined using multistage mass spectrometry experiments in a quadrupole ion trap mass spectrometer. These clusters exhibit size selective reactivity: Ag(2)H(+)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service