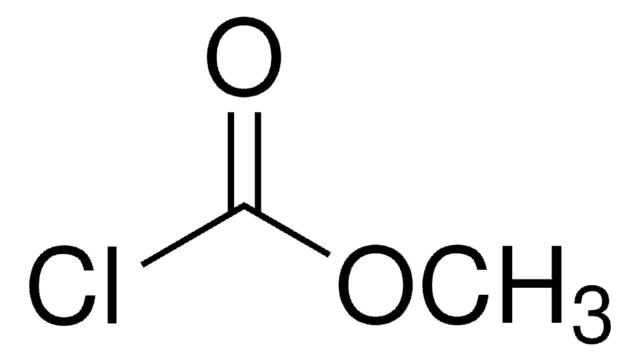
177989
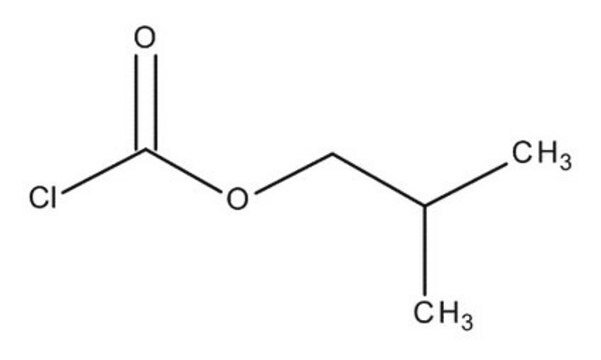
Isobutyl chloroformate
98%
Synonym(s):
Chloroformic acid isobutyl ester, IBCF
About This Item
Recommended Products
vapor pressure
0.33 mmHg ( 20 °C)
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
128.8 °C (lit.)
solubility
benzene: miscible
chloroform: miscible
diethyl ether: miscible
density
1.053 g/mL at 25 °C (lit.)
functional group
chloro
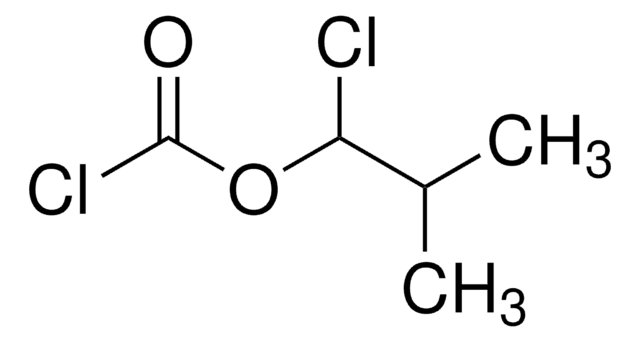
SMILES string
CC(C)COC(Cl)=O
InChI
1S/C5H9ClO2/c1-4(2)3-8-5(6)7/h4H,3H2,1-2H3
InChI key
YOETUEMZNOLGDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Preparation of a volatile derivative of taurine and application to gas chromatographic determination of urinary taurine.: Demonstrates the utility of isobutyl chloroformate in preparing volatile derivatives of taurine for gas chromatographic analysis, offering a methodological advancement in clinical biochemistry (Masuoka et al., 1989).
- Quinazoline antifolates inhibiting thymidylate synthase: synthesis of four oligo(L-gamma-glutamyl) conjugates of N10-propargyl-5,8-dideazafolic acid and their enzyme inhibition.: This article investigates the synthesis and biological activity of quinazoline antifolates, using techniques including isobutyl chloroformate, relevant in medicinal chemistry and drug development (Pawelczak et al., 1989).
- Coupling of peptides to protein carriers by mixed anhydride procedure.: Discusses a novel technique using isobutyl chloroformate for peptide coupling to proteins, useful in bioconjugate chemistry and vaccine development (Samokhin & Filimonov, 1985).
- Folate analogues altered in the C9-N10 bridge region. 18. Synthesis and antitumor evaluation of 11-oxahomoaminopterin and related compounds.: Investigates the role of isobutyl chloroformate in synthesizing folate analogues for cancer research, showing its importance in therapeutic chemistry (Nair et al., 1981).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
96.8 °F - closed cup
Flash Point(C)
36 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service