All Photos(1)
About This Item
Empirical Formula (Hill Notation):
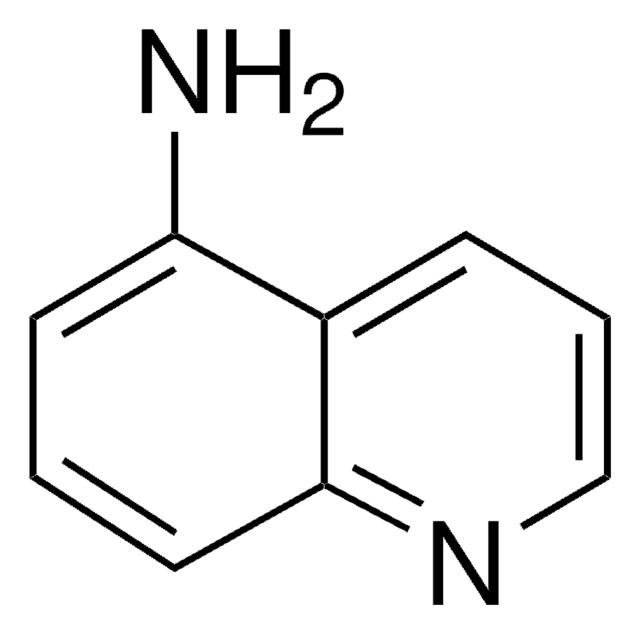
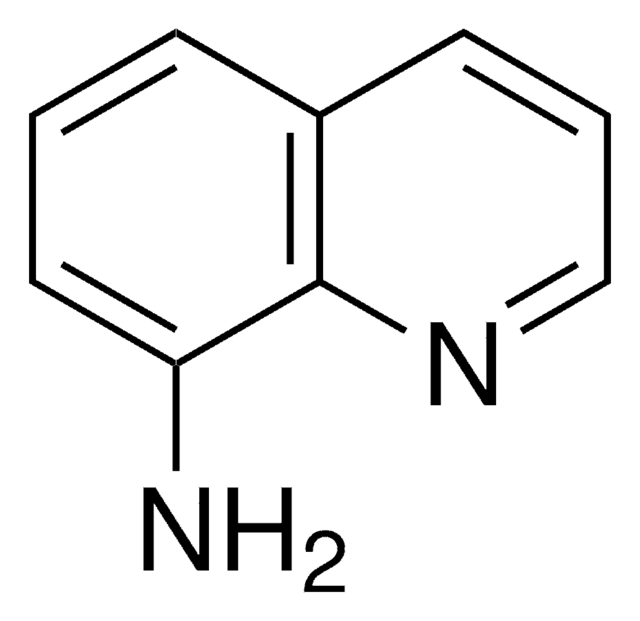
C9H8N2
CAS Number:
Molecular Weight:
144.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
125-128 °C (lit.)
SMILES string
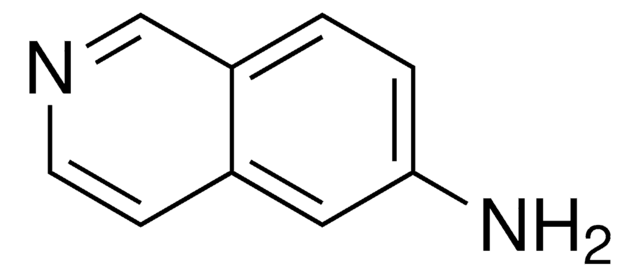
Nc1cccc2cnccc12
InChI
1S/C9H8N2/c10-9-3-1-2-7-6-11-5-4-8(7)9/h1-6H,10H2
InChI key
DTVYNUOOZIKEEX-UHFFFAOYSA-N
General description
5-Aminoisoquinoline forms 1:1 host−guest inclusion complex with β-cyclodextrin. It enhances the chemiluminescence of luminol-H2O2-horseradish peroxidase.
Application
5-Aminoisoquinoline (5AIQ) was used to study the effect of addition of β-cyclodextrin on the absorption and emission properties of 5AIQ.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Host-guest complexation between 5-aminoisoquinoline and ?-cyclodextrin and its effect on spectral and prototropic characteristics.
Rajamohan R, et al.
Journal of Inclusion Phenomena and Macrocyclic Chemistry, 73(1-4), 99-108 (2012)
Relation between the structure of some heterocyclic derivatives and other compounds, and their effects as enhancers or inhibitors of the luminol-H2O2-horseradish peroxidase chemiluminescence.
Garcia SF, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 105(!), 11-14 (1997)
Jürgen Bosch et al.
Journal of medicinal chemistry, 49(20), 5939-5946 (2006-09-29)
The 1.8 A resolution de novo structure of nucleoside 2-deoxyribosyltransferase (EC 2.4.2.6) from Trypanosoma brucei (TbNDRT) has been determined by SADa phasing in an unliganded state and several ligand-bound states. This enzyme is important in the salvage pathway of nucleoside
Sheikh Fayaz Ahmad et al.
Molecular immunology, 63(2), 394-405 (2014-10-12)
Increasing indication is unveiling a role for poly(ADP-ribose) polymerase (PARP)-1 in the regulation of inflammatory/immune responses. The aim of the present study was to determine the potential anti-inflammatory effects of PARP-1 inhibitor 5-aminoisoquinolinone (5-AIQ) to explore the role of PARP-1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service