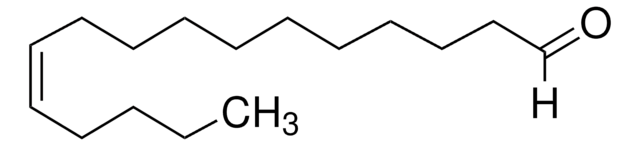
46902-U
cis-9-Octadecenoic acid methyl ester
certified reference material, 10 mg/mL in heptane
Synonym(s):
Oleic acid methyl ester
About This Item
Recommended Products
grade
certified reference material
TraceCERT®
product line
TraceCERT®
form
liquid
CofA
current certificate can be downloaded
packaging
ampule of 1 mL
concentration
10 mg/mL in heptane
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
food and beverages
format
single component solution
functional group
ester
storage temp.
-10 to -25°C
InChI
1S/C19H36O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h10-11H,3-9,12-18H2,1-2H3/b11-10+
InChI key
QYDYPVFESGNLHU-ZHACJKMWSA-N
Application
Other Notes
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Protocols
Separation of Methyl erucate; Methyl palmitate; Methyl stearate; Methyl linolenate; Methyl eicosenoate; Methyl behenate; Methyl myristate; Methyl oleate; Methyl arachidate
Separation of Methyl decanoate; Methyl dodecanoate; Methyl myristate; Methyl palmitate; Methyl caprylate; Methyl oleate; Methyl linoleate; Methyl linolenate; Methyl stearate
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service