P8465
Protease Inhibitor Cocktail
lyophilized powder, for the inhibition of serine, cysteine, aspartic, metalloproteases and aminopeptidases, for use with bacterial cell extracts, lyophilized powder
Synonym(s):
Protease inhibitor
About This Item
Recommended Products
product name
Protease Inhibitor Cocktail powder, for use with bacterial cell extracts, lyophilized powder
Quality Level
form
lyophilized powder
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The product contains individual components that target serine, cysteine, aspartic, and metalloproteases, as well as aminopeptidases.
Application
Biochem/physiol Actions
Features and Benefits
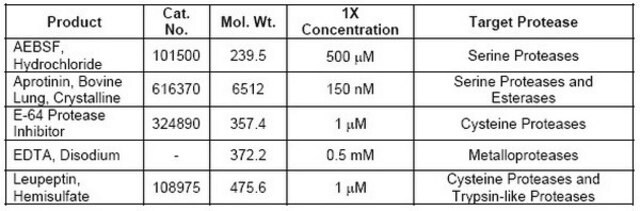
Contains individual components, including AEBSF, EDTA, Bestatin, Pepstatin A, and E-64, each targeting specific types of proteases.
Lyophilized powder is stable for at least 2 years when stored unopened at -20°C.
Supplied with a vial of DMSO for preparation of a cocktail solution.
One mL of the cocktail solution is recommended for the inhibition of protease activity found in 20 mL of cell lysate from 4g of E. coli cells.
Caution
Preparation Note
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A - STOT RE 2 Inhalation
Target Organs
Respiratory Tract
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service