G1251
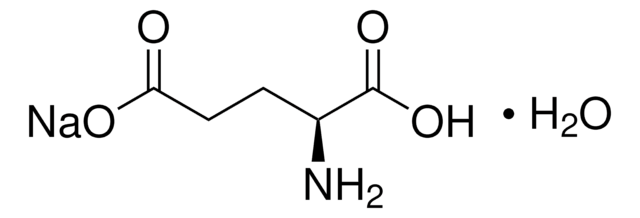
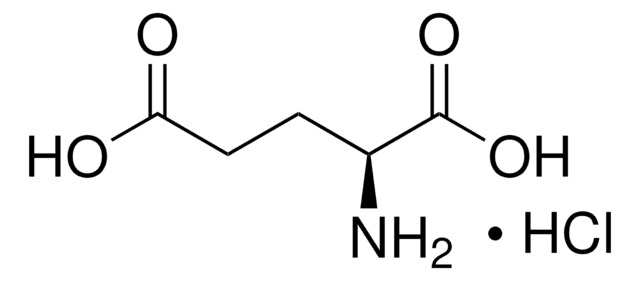
L-Glutamic acid
≥99% (HPLC), suitable for microbiological culture, ReagentPlus®
Synonym(s):
(S)-2-Aminopentanedioic acid, Glu
About This Item
Recommended Products
Product Name
L-Glutamic acid, ReagentPlus®, ≥99% (HPLC)
Agency
suitable for SM 5210
Quality Level
product line
ReagentPlus®
Assay
≥99% (HPLC)
form
powder
technique(s)
microbiological culture: suitable
color
white to off-white
mp
205 °C (dec.) (lit.)
solubility
1 M HCl: soluble 100 mg/mL
density
1.54 g/cm3 at 20 °C
cation traces
C: 40.4-41.2%
N: 9.2-9.8%
SMILES string
N[C@@H](CCC(O)=O)C(O)=O
InChI
1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
InChI key
WHUUTDBJXJRKMK-VKHMYHEASA-N
Gene Information
human ... CCR2(1231) , GRIA1(2890) , GRIA2(2891) , GRIA4(2893) , GRIK2(2898) , GRIK3(2899) , GRIK5(2901) , GRIN2B(2904) , GRM2(2912) , SLC1A1(6505) , SLC1A2(6506)
rat ... Gria1(50592) , Grik1(29559) , Grik2(54257) , Grik4(24406) , Grin2a(24409) , Grm1(24414) , Grm2(24415) , Grm3(24416) , Grm4(24417) , Grm5(24418) , Grm6(24419) , Grm7(81672) , Slc1a2(29482)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Biochem/physiol Actions
Glutamine serves as a source of energy for rapidly dividing cells comprising lymphocytes, enterocytes, macrophages and tumors. Glutamine mediates protein turnover via cellular mTOR (mammalian target of rapamycin) signaling. It is also known to be associated with the inhibition of apoptosis.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service