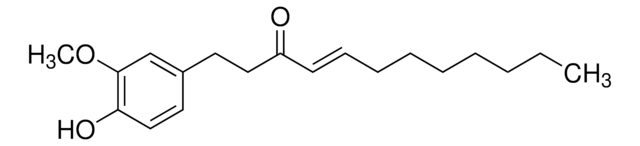
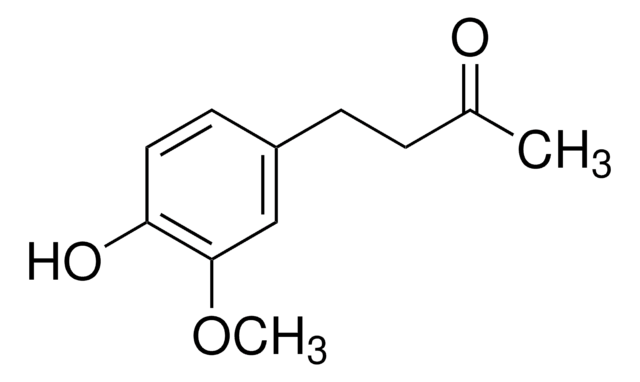
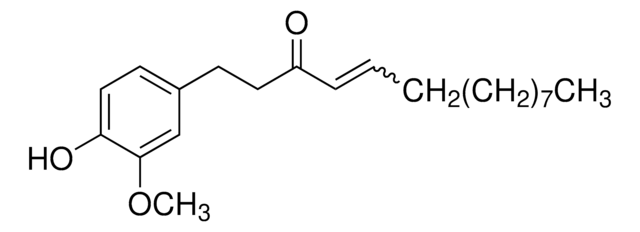
G1046
[6]-Gingerol
≥98% (HPLC)
Synonym(s):
3-Decanone, 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-, (5S)-, 5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone, 6-Gingerol
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
storage condition
protect from light
under inert gas
solubility
methanol: 1 mg/mL, clear, colorless
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCC[C@H](O)CC(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H26O4/c1-3-4-5-6-14(18)12-15(19)9-7-13-8-10-16(20)17(11-13)21-2/h8,10-11,14,18,20H,3-7,9,12H2,1-2H3/t14-/m0/s1
InChI key
NLDDIKRKFXEWBK-AWEZNQCLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- to study its effects on transient receptor potential (TRP) channels
- to study its effects on experimental models of non-alcoholic steatohepatitis
- to determine its effects on microsomal prostaglandine E2 synthase 1 (mPGES-1), glycogen synthase kinase 3β (GSK-3β) and β-catenin pathway in A549 cell line
- to analyse the effects of 6-Shogaol (6-SG) on diabetic nephropathy (DN) in db/db mice
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
NF-κB and Inflammation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)
![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)
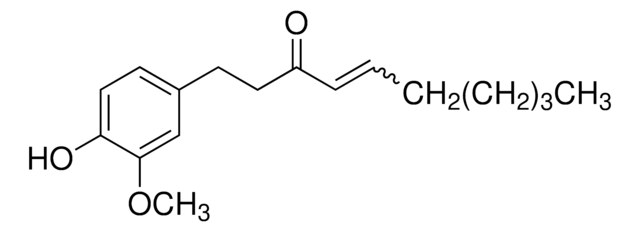

![[8]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/408/530/af2f2837-3419-4e07-a72b-24e95af0d7ce/640/af2f2837-3419-4e07-a72b-24e95af0d7ce.png)
![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)