B1403
Boc-L-alaninal
≥98%
Synonym(s):
Boc-L-alanine aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H15NO3
CAS Number:
Molecular Weight:
173.21
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Product Name
Boc-L-alaninal, ≥98%
Quality Level
Assay
≥98%
form
powder
color
white
application(s)
peptide synthesis
storage temp.
−20°C
SMILES string
C[C@H](NC(=O)OC(C)(C)C)C=O
InChI
1S/C8H15NO3/c1-6(5-10)9-7(11)12-8(2,3)4/h5-6H,1-4H3,(H,9,11)/t6-/m0/s1
InChI key
OEQRZPWMXXJEKU-LURJTMIESA-N
Gene Information
human ... CTSK(1513)
Related Categories
Application
Boc-L-alaninal is used as a reagent for organic synthesis of C(26)-C(32) Oxazole Fragment of Calyculin C and other molecules.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Petri M. Pihko et al.
The Journal of organic chemistry, 63(1), 92-98 (2001-10-25)
The synthesis of the C(26)-C(32) oxazole fragment 4 and its C(32) epimer 20 of serine/threonine protein phosphatase PP1 and PP2A inhibitor calyculin C is presented. The syn methyl arrangement in 4 was established through cyclic stereocontrol. Several methods for oxidizing
Johann Chan et al.
The Journal of organic chemistry, 76(6), 1767-1774 (2011-02-09)
Two new, reliable syntheses of a pyrido[2,3-d]-pyrimidine inhibitor of the CXCR3 receptor are described. A nine-step synthesis of the CXCR3 inhibitor (1) from 2-aminonicotinic acid was demonstrated on a multikilogram scale and incorporates a classic resolution to deliver the enantioenriched
James A Marshall et al.
Organic letters, 7(8), 1593-1596 (2005-04-09)
[reaction: see text] Additions of chiral allenylzinc and indium reagents to N-Boc alaninal were examined as a possible route to a C20-C26 segment of superstolide A. Allenylzinc reagents, prepared in situ by palladiozincation of (R)- and (S)-5-pivalyloxy-3-butyn-2-ol mesylate, showed excellent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service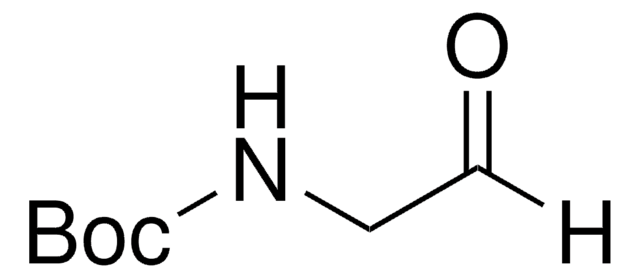
![3-[(Benzyloxycarbonyl)amino]propionaldehyde 95%](/deepweb/assets/sigmaaldrich/product/structures/408/203/100fb0f0-7072-41be-b6e0-2857cdc324ee/640/100fb0f0-7072-41be-b6e0-2857cdc324ee.png)