92841
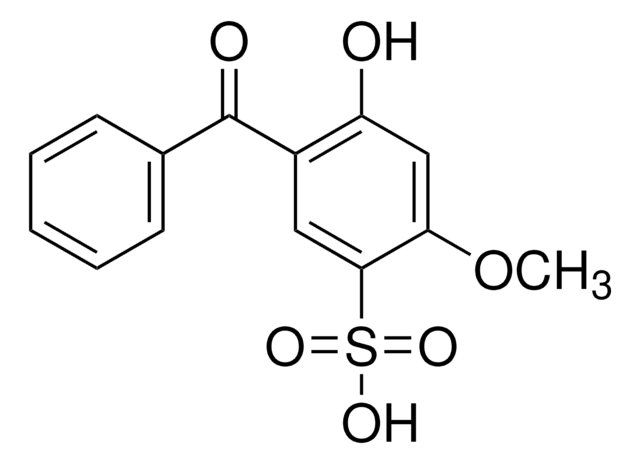
Dioxybenzone
analytical standard
Synonym(s):
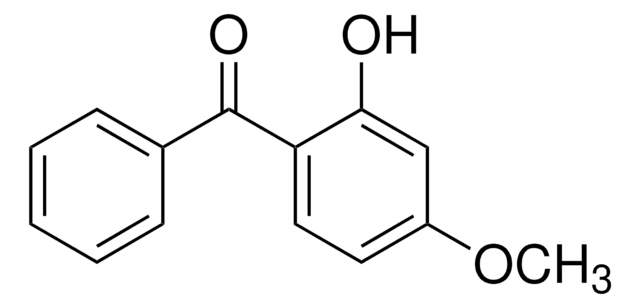
2,2′-Dihydroxy-4-methoxybenzophenone, Benzophenone-8, Dioxybenzone
About This Item
Recommended Products
grade
analytical standard
Quality Level
Assay
≥98.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
bp
170-175 °C/1 mmHg (lit.)
mp
72-74 °C
73-75 °C (lit.)
application(s)
cleaning products
cosmetics
environmental
food and beverages
personal care
format
neat
SMILES string
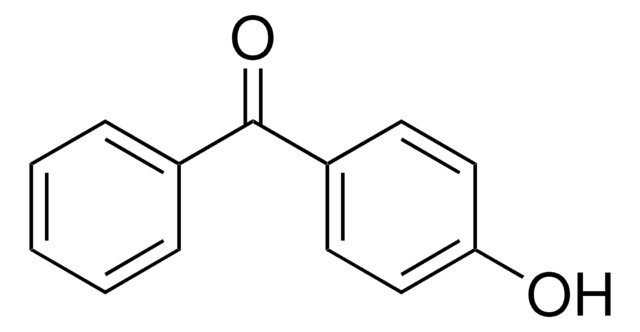
COc1ccc(c(O)c1)C(=O)c2ccccc2O
InChI
1S/C14H12O4/c1-18-9-6-7-11(13(16)8-9)14(17)10-4-2-3-5-12(10)15/h2-8,15-16H,1H3
InChI key
MEZZCSHVIGVWFI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
Packaging
Other Notes
The collision cross section (CCS) measurement was provided by Waters Corporation, using the SYNAPT XS mass spectrometer.
For a description and overview of how ion mobility enables the measurement of the CCS of an ion visit ims.waters.com.
Further information on the SYNAPT XS mass spectrometer can be found on the IMS microsite and product webpage.
TWCCS measurements are expected to be within 2% of this reference value.
P/N 92841 is part of the Waters Extractables & Leachables UNIFI scientific library which can be downloaded from Waters Marketplace.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service