17755
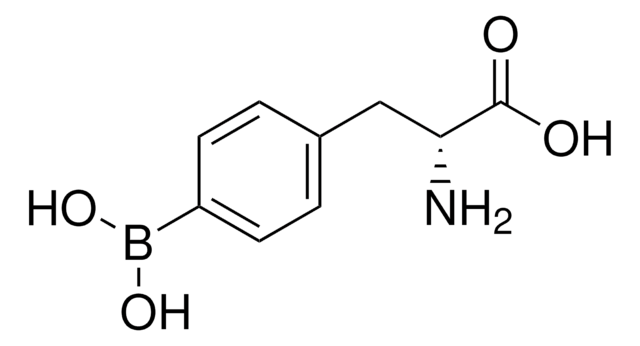
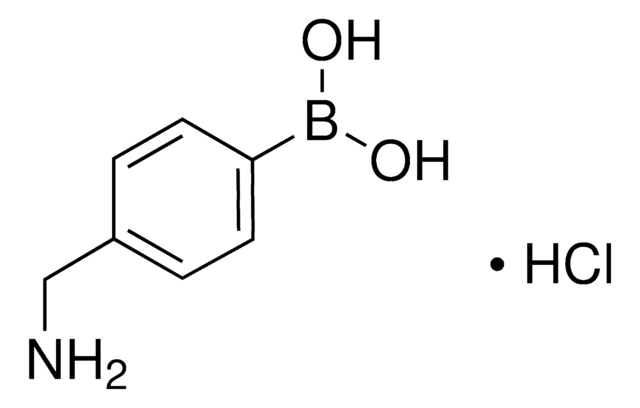
4-Borono-L-phenylalanine
≥95.0% (HPLC)
Synonym(s):
4-Dihydroxyboryl-L-phenylalanine, L-BPA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12BNO4
CAS Number:
Molecular Weight:
209.01
Beilstein:
4458616
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95.0% (HPLC)
storage temp.
2-8°C
SMILES string
N[C@@H](Cc1ccc(cc1)B(O)O)C(O)=O
InChI
1S/C9H12BNO4/c11-8(9(12)13)5-6-1-3-7(4-2-6)10(14)15/h1-4,8,14-15H,5,11H2,(H,12,13)/t8-/m0/s1
InChI key
NFIVJOSXJDORSP-QMMMGPOBSA-N
Application
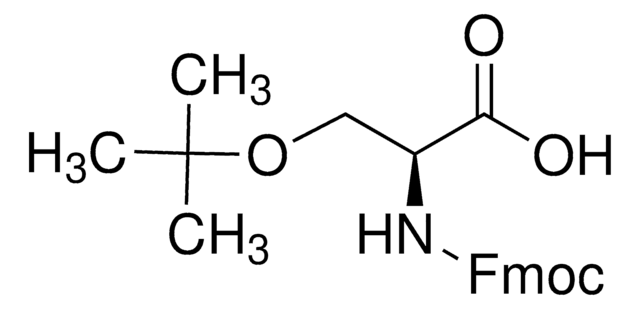
4-Borono-L-phenylalanine can be used as a building block in solid-phase peptide synthesis. It can also be used to synthesize substituted triazine derivatives as potential tryptophan hydroxylase inhibitors via Suzuki cross-coupling reaction using palladium as a catalyst.
Other Notes
Tyrosine analogue; employed for treatment of melanom cells by boron neutron capture therapy
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Romina F Aromando et al.
Oral oncology, 46(5), 355-359 (2010-03-24)
Mast cell (MC) activation in the hamster cheek pouch cancerization model is associated with the increase in tumor cell proliferation, mediated in turn by tryptase, a protease released from mast cell granules after activation. Tryptase induces tumor cell proliferation through
T Fujimoto et al.
Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine, 69(12), 1713-1716 (2011-03-01)
Clear cell sarcoma (CCS), a rare malignant tumor with a predilection for young adults, is of poor prognosis. Recently however, boron neutron capture therapy (BNCT) with the use of p-borono-L-phenylalanine (BPA) for malignant melanoma has provided good results. CCS also
Ana J Molinari et al.
Radiation research, 177(1), 59-68 (2011-10-11)
We previously demonstrated the efficacy of BNCT mediated by boronophenylalanine (BPA) to treat tumors in a hamster cheek pouch model of oral cancer with no normal tissue radiotoxicity and moderate, albeit reversible, mucositis in precancerous tissue around treated tumors. It
Andrea Wittig et al.
Radiation research, 172(4), 493-499 (2009-09-24)
In boron neutron capture therapy, the absorbed dose from the (10)B(n,alpha)(7)Li reaction depends on the (10)B concentration and (10)B distribution in the irradiated volume. Thus compounds used in BNCT should have tumor-specific uptake and low accumulation in normal tissues. This
Tsubasa Watanabe et al.
BMC cancer, 16(1), 859-859 (2016-11-09)
Boron neutron capture therapy (BNCT) is a cellular-level particle radiation therapy that combines the selective delivery of boron compounds to tumour tissue with neutron irradiation. L-p-Boronophenylalanine (L-BPA) is a boron compound now widely used in clinical situations. Determination of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service