07-4770
Dimethyl phthalate
SAJ special grade, ≥99.0%
Synonym(s):
DMP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
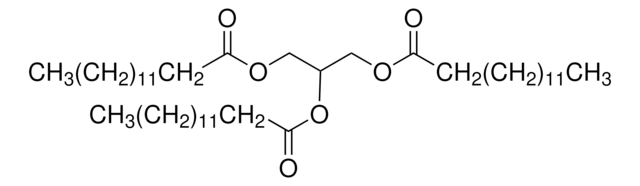
C6H4-1,2-(CO2CH3)2
CAS Number:
Molecular Weight:
194.18
Beilstein:
1911460
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
Recommended Products
grade
SAJ special grade
Assay
≥99.0%
form
liquid
autoignition temp.
1033 °F
availability
available only in Japan
refractive index
n20/D 1.515 (lit.)
bp
282 °C (lit.)
mp
2 °C (lit.)
density
1.19 g/mL at 25 °C (lit.)
SMILES string
COC(=O)c1ccccc1C(=O)OC
InChI
1S/C10H10O4/c1-13-9(11)7-5-3-4-6-8(7)10(12)14-2/h3-6H,1-2H3
InChI key
NIQCNGHVCWTJSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
294.8 °F - closed cup
Flash Point(C)
146.0 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhengwen Xu et al.
Environmental science & technology, 44(8), 3130-3135 (2010-03-17)
In the present study, a novel approach was developed to remove dimethyl phthalate (DMP), a representative phthalic acid ester (PAE) pollutant, from an aqueous solution using a macroporous OH-type strong base anion exchange resin D201-OH. As compared to the traditional
L J Xu et al.
Water research, 47(6), 1996-2004 (2013-02-12)
The merits of the combined process of high-frequency ultrasound (US) and catalyst-free ultraviolet irradiation (UV) have been evaluated in this study by investigating the sonophotolytic degradation of dimethyl phthalate (DMP). A 400 kHz ultrasonic system and a photolytic system at
L J Xu et al.
Ultrasonics sonochemistry, 20(3), 892-899 (2012-12-05)
A comprehensive study of the sonochemical degradation of dimethyl phthalate (DMP) was carried out using high-frequency ultrasonic processes. The effects of various operating parameters were investigated, including ultrasonic frequency, power density, initial DMP concentration, solution pH and the presence of
Yiling Chen et al.
Journal of hazardous materials, 235-236, 92-100 (2012-08-14)
In this study, we demonstrate that the decomposition of dimethyl phthalate under microwave irradiation could be greatly enhanced over Fe@Fe(2)O(3) nanowires supported on activated carbon (Fe@Fe(2)O(3)/AC). The great enhanced decomposition of dimethyl phthalate could be attributed to a unique microwave
Yi-Hung Chen et al.
Journal of hazardous materials, 192(3), 1017-1025 (2011-07-05)
The removal of dimethyl phthalate (DMP), which is a pollutant of concern in water environments, was carried out by catalytic ozonation with TiO(2)/Al(2)O(3) catalysts. The heterogeneous catalytic ozonation was an ozonation process combined with the catalytic and adsorptive properties of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service