FCMAB323PE
Milli-Mark® Anti-Phosphotyrosine-PE Antibody, recombinant clone 4G10®
clone 4G10, Milli-Mark®, from mouse
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
conjugate
PE
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
4G10, monoclonal
species reactivity
vertebrates, human
manufacturer/tradename
Milli-Mark®
technique(s)
flow cytometry: suitable
isotype
IgG2bκ
shipped in
wet ice
target post-translational modification
phosphorylation (pTyr)
Gene Information
human ... PID1(55022)
General description
Some of the tyrosine residues can be tagged with a phosphate group by protein kinases and in its phosphorylated state, it is referred to as phosphotyrosine. Tyrosine phosphorylation is considered as one of the key steps in signal transduction and regulation of enzymatic activity.
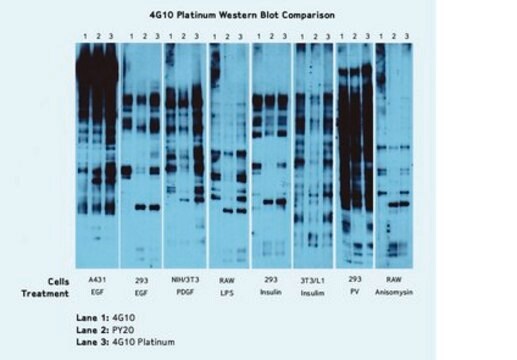
The advent of anti-phosphotyrosine antibodies is one of the significant events in signal transduction research. Before the availability of anti-phosphotyrosine antibodies, tyrosine phosphorylation of proteins and enzymes was investigated through hazardous and time-consuming radioactive experiments. Anti-phosphotyrosine antibodies are commonly used in western blots after the targeted proteins have been immunoprecipitated to measure the tyrosyne phosphorylation of the proteins. Anti-phosphotyrosine antibodies are also directly used on cell lysate to examine the overall change of tyrosine phosphorylation level in reponse to various treatments.
The advent of anti-phosphotyrosine antibodies is one of the significant events in signal transduction research. Before the availability of anti-phosphotyrosine antibodies, tyrosine phosphorylation of proteins and enzymes was investigated through hazardous and time-consuming radioactive experiments. Anti-phosphotyrosine antibodies are commonly used in western blots after the targeted proteins have been immunoprecipitated to measure the tyrosyne phosphorylation of the proteins. Anti-phosphotyrosine antibodies are also directly used on cell lysate to examine the overall change of tyrosine phosphorylation level in reponse to various treatments.
Specificity
Antibody recognizes tyrosine-phosphorylated proteins from all species
Expected to detect tyrosyne phosphorylated proteins in all species.
Application
Milli-Mark Anti-Phosphotyrosine-PE Antibody, recombinant clone 4G10 is an antibody against Phosphotyrosine clone 4G10 for use in FC.
Quality
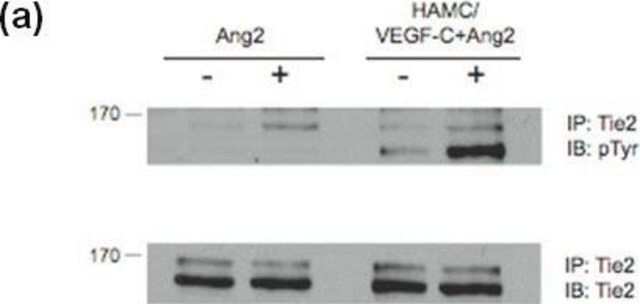
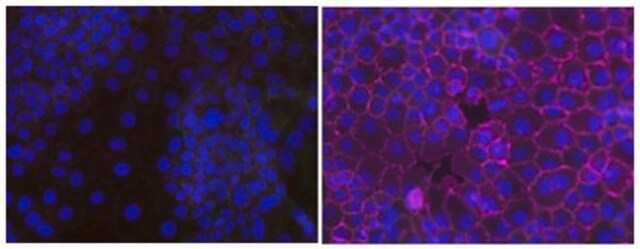
Evaluated by flow cytometry using EGF treated versus untreated A431 cells
Target description
Dependent upon the molecular weight of the tyrosine phosphorylated protein being detected.
Physical form
Purified mouse monoclonal IgG2bk conjugated to PE in PBS with 0.1% sodium azide and 15 mg/mL BSA.
Analysis Note
Control
Treated versus untreated A431 cells
Treated versus untreated A431 cells
Legal Information
4G10 is a registered trademark of Upstate Group, Inc.
MILLI-MARK is a registered trademark of Merck KGaA, Darmstadt, Germany
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Veronika Horkova et al.
Cell reports, 30(5), 1504-1514 (2020-02-06)
Overtly self-reactive T cells are removed during thymic selection. However, it has been recently established that T cell self-reactivity promotes protective immune responses. Apparently, the level of self-reactivity of mature T cells must be tightly balanced. Our mathematical model and experimental data
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service