700081P
Avanti
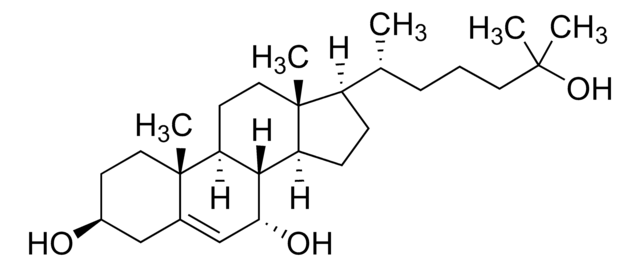
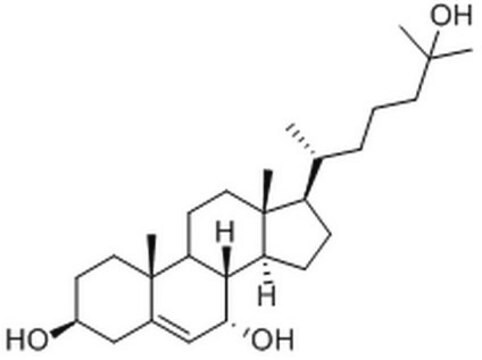
7β,25-dihydroxycholesterol
Avanti Research™ - A Croda Brand
Synonym(s):
cholest-5-ene-3β,7β,25-triol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C27H46O3
CAS Number:
Molecular Weight:
418.65
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 1 mg (700081P-1mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand
shipped in
dry ice
storage temp.
−20°C
SMILES string
CC1(CC[C@H](O)C2)C2=C[C@H](O)C3C1CCC4(C)C3CCC4C(CCCC(C)(O)C)C
General description
7β,25-dihydroxycholesterol (7β25OHC) is an isomer of 7α,25-dihydroxycholesterol (7α25OHC). Its synthesis from 7-keto,25-hydroxycholesterol (7k25OHC) is catalyzed by 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1). 11β-Hydroxysteroid dehydrogenase type 2 (11β-HSD2) catalyzes the reverse oxidation from 7β25OHC to 7k25OHC.
Application
7β,25-dihydroxycholesterol has been used as a substrate for 11β-Hydroxysteroid dehydrogenase (11β-HSD)-dependent metabolic studies of oxysterols in human embryonic kidney (HEK-293) cells in enzyme kinetics assay and as a standard in high-performance liquid chromatography(HPLC) for detection of dihydroxycholesterols in human plasma.
Biochem/physiol Actions
7β,25-dihydroxycholesterol is an Epstein-Barr virus-induced gene 2 (EBI2) ligand and is comparatively a weaker chemoattractant than 7α,25-dihydroxycholesterol (7α25OHC).
Packaging
5 mL Amber Glass Screw Cap Vial (700081P-1mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Katharina R Beck et al.
The Journal of steroid biochemistry and molecular biology, 190, 19-28 (2019-03-25)
Oxysterols are cholesterol metabolites derived through either autoxidation or enzymatic processes. They consist of a large family of bioactive lipids that have been associated with the progression of multiple pathologies. In order to unravel (patho-)physiological mechanisms involving oxysterols, it is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service