T46108
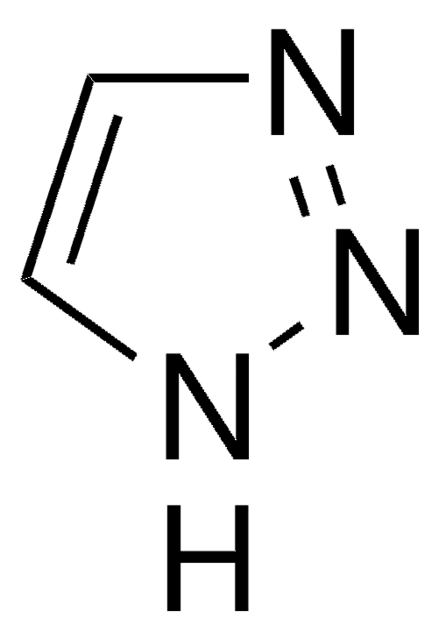
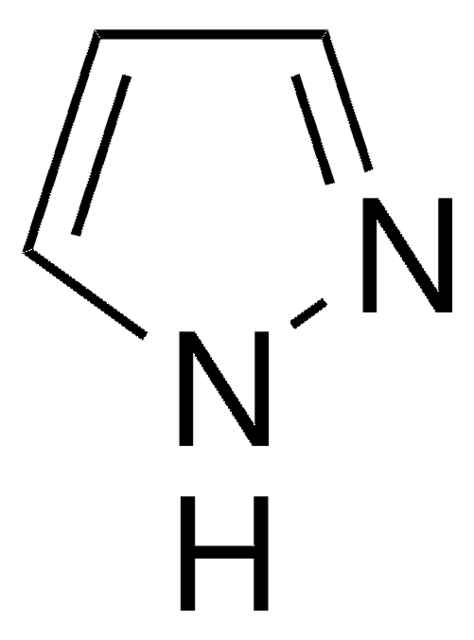
1,2,4-Triazole
98%
Synonym(s):
3,4-Diazapyrrole, 4H-1,2,4-Triazole, s-Triazole (8CI)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C2H3N3
CAS Number:
Molecular Weight:
69.07
Beilstein:
104767
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
260 °C (lit.)
mp
119-121 °C (lit.)
SMILES string
c1nc[nH]n1
InChI
1S/C2H3N3/c1-3-2-5-4-1/h1-2H,(H,3,4,5)
InChI key
NSPMIYGKQJPBQR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,2,4-triazole and its derivatives are important structural moieties of many pharmaceutical drugs. Triazoles can also act as ligands to form coordination complexes with transition metal ions. Due to their electron-deficient nature, they exhibit excellent electron-transport and hole-blocking properties, making them promising organic materials in material science applications.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
338.0 °F
Flash Point(C)
170 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Applications of Metal-Free 1, 2, 4-Triazole Derivatives in Materials Science.
Diaz-Ortiz A
Current Organic Chemistry, 19(7), 568-584 (2015)
Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1, 2, 4-triazole derivatives as ligands.
Haasnoot JG
Coordination Chemistry Reviews, 200, 131-185 (2000)
1, 2, 4-Triazoles: Synthetic approaches and pharmacological importance.
Al-Masoudi IA
Chemistry of Heterocyclic Compounds, 42(11), 1377-1403 (2006)
Margaret E Olson et al.
ChemMedChem, 8(1), 112-117 (2012-11-28)
APOBEC3G (A3G) is a single-stranded DNA cytosine deaminase that functions in innate immunity against retroviruses and retrotransposons. Although A3G can potently restrict Vif-deficient HIV-1 replication by catalyzing excessive levels of G→A hypermutation, sublethal levels of A3G-catalyzed mutation may contribute to
Tomasz Plech et al.
European journal of medicinal chemistry, 60, 208-215 (2013-01-08)
Designed and synthesized 4-alkyl-1,2,4-triazole-3-thione derivatives showed significant anticonvulsant activity, determined in the maximal electroshock-induced seizure (MES) test. The chemical structure of all new compounds was confirmed by spectral methods ((1)H NMR, (13)C NMR, IR, MS). A sensitive and selective method
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service