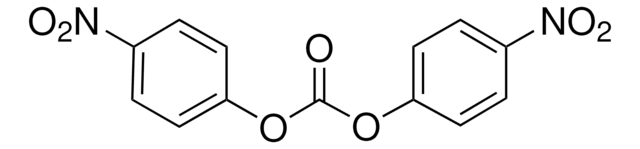
N21022
4-Nitrophenyl disulfide
Synonym(s):
p,p′-Dinitrodiphenyl disulfide, Bis(4-nitrophenyl) disulfide, Bis(p-nitrophenyl) disulfide, NSC 4566, NSC 677446
About This Item
Recommended Products
form
powder
Quality Level
SMILES string
[O-][N+](=O)c1ccc(SSc2ccc(cc2)[N+]([O-])=O)cc1
InChI
1S/C12H8N2O4S2/c15-13(16)9-1-5-11(6-2-9)19-20-12-7-3-10(4-8-12)14(17)18/h1-8H
InChI key
KWGZRLZJBLEVFZ-UHFFFAOYSA-N
Application
- Electrophilic cyclization of 2-alkynylanisoles or alkynylanilines
- Oxidative chlorination to sulfonyl chlorides
- Arylation with triarylbismuthanes
- Decarboxylative cross-coupling with dialkoxybenzoic acids
- Disulfidation of alkenes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
While Markovnikov alkene reactivity is very well developed and utilized commonly in the synthesis of commodity and research chemicals, catalytic access to the anti-Markovnikov-selective adducts is a much less-developed endeavor.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service