M29355
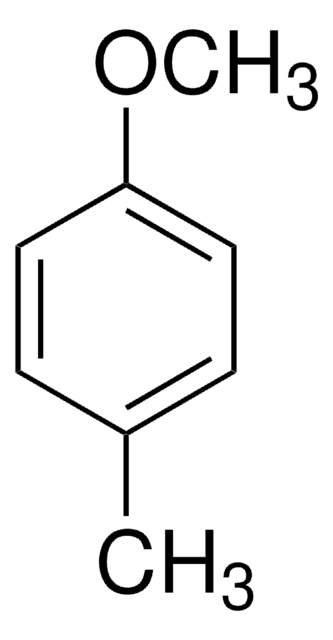
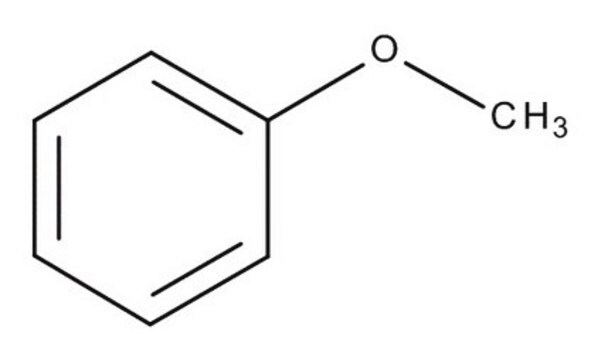
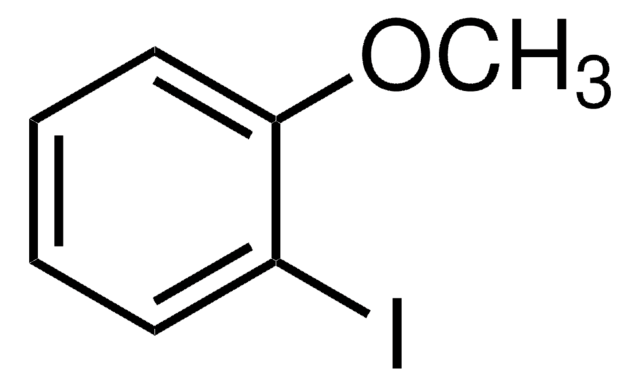
2-Methylanisole
99%
Synonym(s):
2-Methyl-1-methoxybenzene, o-Cresol methyl ether, o-Cresyl methyl ether, o-Methoxytoluene, o-Methylanisole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H4OCH3
CAS Number:
Molecular Weight:
122.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.516 (lit.)
bp
170-172 °C (lit.)
density
0.985 g/mL at 25 °C (lit.)
SMILES string
COc1ccccc1C
InChI
1S/C8H10O/c1-7-5-3-4-6-8(7)9-2/h3-6H,1-2H3
InChI key
DTFKRVXLBCAIOZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Graziela G Bianco et al.
The Journal of organic chemistry, 74(6), 2561-2566 (2009-02-24)
The first synthesis of the natural product (+)-mutisianthol was accomplished in 11 steps and in 21% overall yield from 2-methylanisole. The synthesis of its enantiomer was also performed in a similar overall yield. The absolute configuration of the sesquiterpene (+)-mutisianthol
Elodie Guyonnet Bilé et al.
ChemSusChem, 5(1), 91-101 (2012-01-18)
Optically active amphiphilic compounds derived from N-methylephedrine, N-methylprolinol, or cinchona derivatives possessing bromide or chiral lactate counterions were efficiently used as protective agents for rhodium(0) nanoparticles. The full characterization of these surfactants and the obtained nanocatalysts was performed by means
L Higgins et al.
Archives of biochemistry and biophysics, 385(1), 220-230 (2001-05-22)
Regioselectivity is used to determine the absolute energetic differences for four different reactions catalyzed by P450. Abstraction of a hydrogen from a benzylic carbon containing a chlorine has a 1.0 kcal/mol lower barrier than abstraction from a simple benzylic carbon
Johannes Niebler et al.
Phytochemistry, 109, 66-75 (2014-12-04)
Frankincense has been known, traded and used throughout the ages for its exceptional aroma properties, and is still commonly used in both secular and religious settings to convey a pleasant odor. Surprisingly, the odoriferous principle(s) underlying its unique odor profile
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service