86885
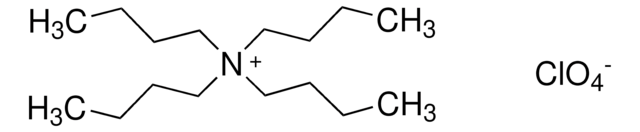
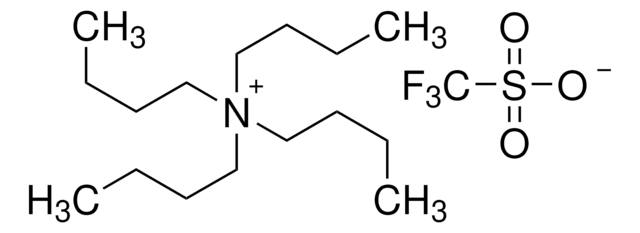
Tetrabutylammonium perchlorate
≥95.0% (T)
Synonym(s):
TBAP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
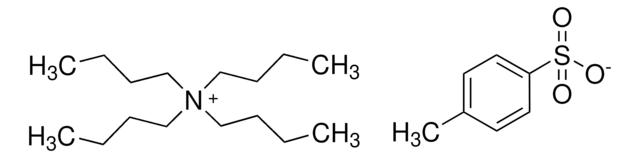
(CH3CH2CH2CH2)4N(ClO4)
CAS Number:
Molecular Weight:
341.91
Beilstein:
3579274
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0% (T)
form
powder
reaction suitability
reagent type: oxidant
SMILES string
[O-]Cl(=O)(=O)=O.CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.ClHO4/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;2-1(3,4)5/h5-16H2,1-4H3;(H,2,3,4,5)/q+1;/p-1
InChI key
KBLZDCFTQSIIOH-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Tetrabutylammonium perchlorate is a tetraalkylammonium salt that can be used in phase-transfer catalysis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
ESCA studies of phase-transfer catalysts in solution: ion pairing and surface activity
Moberg R, et al.
Journal of the American Chemical Society, 113(10), 3663-3667 (1991)
David Balcells et al.
Dalton transactions (Cambridge, England : 2003), (30)(30), 5989-6000 (2009-07-23)
Experimental studies have shown that the C-H oxidation of Ibuprofen and methylcyclohexane acetic acid can be carried out with high selectivities using [(terpy')Mn(OH(2))(mu-O)(2)Mn(OH(2))(terpy')](3+) as catalyst, where terpy' is a terpyridine ligand functionalized with a phenylene linker and a Kemp's triacid
Atanu Jana et al.
Dalton transactions (Cambridge, England : 2003), 44(1), 359-367 (2014-11-11)
A novel electron rich, tetrathiafulvalene fused zinc porphyrin, (TTF)4PZn, has been newly synthesized and characterized using spectral and electrochemical methods. In spite of the presence of eight t-butyl groups, (TTF)4PZn exhibited appreciable aggregation in solution. Scanning electron microscopic (SEM) imaging
R Bryan Sears et al.
Journal of inorganic biochemistry, 121, 77-87 (2013-01-29)
The complex cis-[Ru(phpy)(phen)(CH3CN)2](+) (phpy=2-phenylpyridine, phen=1,10-phenanthroline) was investigated as a potential photodynamic therapy (PDT) agent. This complex presents desirable photochemical characteristics including a low energy absorption tail extending into the PDT window (600-850nm) and photoinduced exchange of the CH3CN ligands, generating
Oliver Bixner et al.
The Journal of chemical physics, 136(20), 204503-204503 (2012-06-07)
The interaction of exciton and charge transfer (CT) states plays a central role in photo-induced CT processes in chemistry, biology, and physics. In this work, we use a combination of two-dimensional electronic spectroscopy (2D-ES), pump-probe measurements, and quantum chemistry to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service