776505
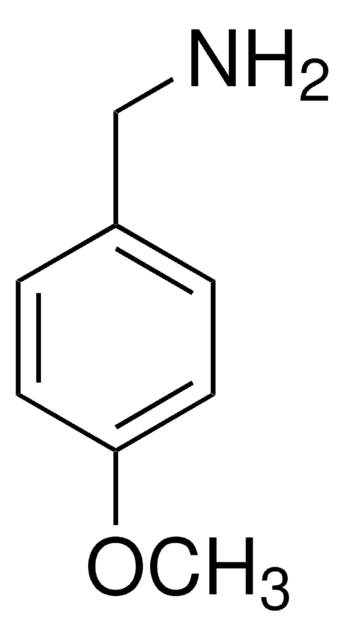
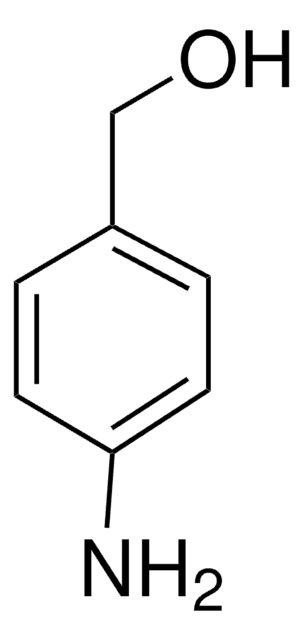
4-Hydroxybenzylamine
Synonym(s):
4-(Aminomethyl)phenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H9NO
CAS Number:
Molecular Weight:
123.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
mp
123-128 °C
functional group
amine
SMILES string
NCc1ccc(O)cc1
InChI
1S/C7H9NO/c8-5-6-1-3-7(9)4-2-6/h1-4,9H,5,8H2
InChI key
RQJDUEKERVZLLU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Hartmann et al.
Biochemistry, 32(9), 2234-2241 (1993-03-09)
Anaerobic, rapid-scanning stopped-flow spectroscopy has been used to investigate the UV-visible absorbance changes (300-540 nm) that occur in the spectrum of bovine serum amine oxidase during reduction by benzylamine, p-hydroxybenzylamine, and p-methoxybenzylamine. The reaction of enzyme with benzylamine generates detectable
P R Williamson et al.
The Journal of biological chemistry, 261(20), 9477-9482 (1986-07-15)
The catalysis of amine oxidation by lysyl oxidase has been probed to assess for the likely order of substrate binding and product release and to discriminate between mechanistic alternatives previously proposed for other copper-dependent amine oxidases using molecular oxygen as
Qichen Zhan et al.
Small (Weinheim an der Bergstrasse, Germany), 15(3), e1803926-e1803926 (2018-11-30)
Controlled drug release systems can enhance the safety and availability but avoid the side effect of drugs. Herein, the concept of DNA complementary base pairing rules in biology is used to design and prepare a photothermal-triggered drug release system. Adenine
Kine S N Dervola et al.
Neurotoxicology, 50, 38-45 (2015-07-29)
Polychlorinated biphenyls (PCBs) are present as ortho- and non-ortho-substituted PCBs, with most of the ortho-substituted congeners being neurotoxic. The present study examined effects of the ortho-substituted PCB 153 on dopamine, serotonin and amino acid neurotransmitters in the neostriatum of both
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 776505-5G | 4061832940403 |
| 776505-25G | 4061832940397 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service