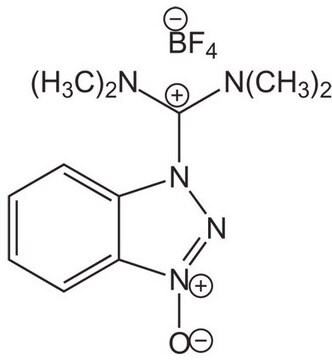
749613
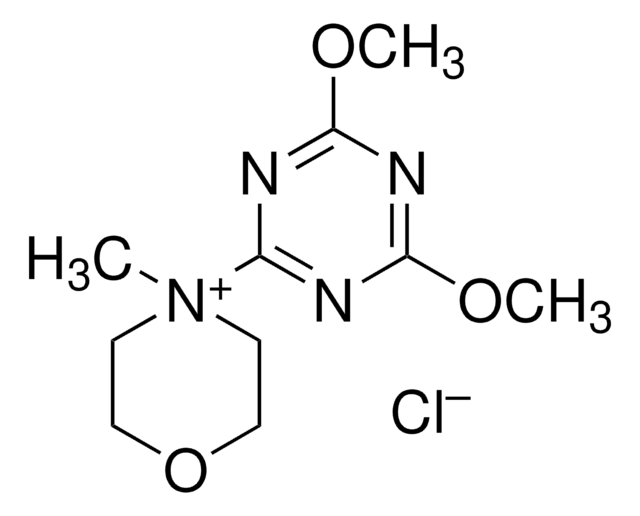
4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate
97%
Synonym(s):
DMTMM, MMTM
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H17BF4N4O3
Molecular Weight:
328.07
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
202-207 °C
functional group
ether
SMILES string
F[B-](F)(F)F.COc1nc(OC)nc(n1)[N+]2(C)CCOCC2
InChI
1S/C10H17N4O3.BF4/c1-14(4-6-17-7-5-14)8-11-9(15-2)13-10(12-8)16-3;2-1(3,4)5/h4-7H2,1-3H3;/q+1;-1
InChI key
LCRXDNPNQMIQFZ-UHFFFAOYSA-N
Application
4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate (DMTMM BF4) can be used as a coupling reagent alternative to DMTMM Cl in peptide synthesis because of its stability. It is also used in the synthesis of:
- Esters and peptides in both solution and solid-phase.
- γ-aminobutyric acid (GABA)-containing cyclic heptapeptide, unguisin A.
- The cyclization of linear tetrapeptides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
George Lo Huang et al.
Nanomaterials (Basel, Switzerland), 9(5) (2019-05-12)
Indoleamine 2,3-dioxygenase (IDO) is an immunomodulating enzyme that is overexpressed in many cancers with poor prognosis. IDO suppresses T cell immunity by catabolizing tryptophan into kynurenine (KYN), which induces apoptosis in T effector cells and enhances T regulatory cells, providing
Xiaoqin Zhang et al.
Carbohydrate polymers, 247, 116749-116749 (2020-08-25)
To enhance the drug delivery efficiency of hyaluronic acid (HA), we designed and prepared glycodendron and pyropheophorbide-a (Ppa)-functionalized HA (HA-Ppa-Dendron) as a nanosystem for cancer photodynamic therapy. Linear Ppa-modified HA (HA-Ppa) was also prepared as a control. Cellular uptake of
An improved process for the synthesis of DMTMM-based coupling reagents
Raw SA
Tetrahedron Letters, 50(8), 946-948 (2009)
Total synthesis of unguisin A
Hunter L and Chung JH
The Journal of Organic Chemistry, 76(13), 5502-5505 (2011)
Maria Laura Alfieri et al.
Antioxidants (Basel, Switzerland), 9(3) (2020-03-22)
The ability of gelatin-based hydrogels of incorporating and releasing under controlled conditions 5,6-dihydroxyindole-2-carboxylic acid (DHICA), a melanin-related metabolite endowed with marked antioxidant properties was investigated. The methyl ester of DHICA, MeDHICA, was also tested in view of its higher stability
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service