690953
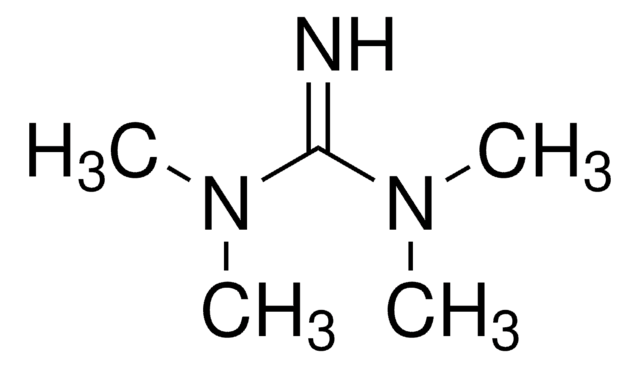
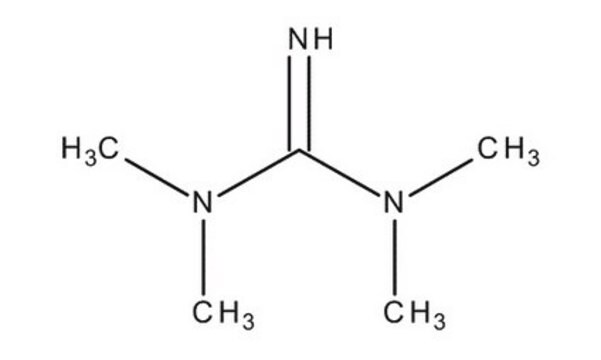
N,N,N′,N′-Tetramethylguanidine
≥99.0% (GC)
Synonym(s):
1,1,3,3-Tetramethylguanidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NC(=NH)N(CH3)2
CAS Number:
Molecular Weight:
115.18
Beilstein:
969608
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99.0% (GC)
form
liquid
impurities
≤0.50% water
color
APHA: ≤150
bp
162-163 °C (lit.)
density
0.916 g/mL at 20 °C (lit.)
functional group
amine
SMILES string
CN(C)C(=N)N(C)C
InChI
1S/C5H13N3/c1-7(2)5(6)8(3)4/h6H,1-4H3
InChI key
KYVBNYUBXIEUFW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N,N,N′,N′-Tetramethylguanidine can be used to synthesize:
- dinucleoside phosphotriester1
- 1,1,3,3-tetramethylguanidinium 2-ethylhexoate2
- tetramethylguanidine ionic liquid (TMG IL)3
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Met. Corr. 1 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetramethyl guanidinium chlorosulfonate as a highly efficient and recyclable organocatalyst for the preparation of bis (indolyl) methane derivatives.
Kalla R, et al.
Catalysis Communications, 57, 55-59 (2014)
Deoxyribonucleoside cyclic N-acylphosphoramidites as a new class of monomers for the stereocontrolled synthesis of oligothymidylyl-and oligodeoxycytidylyl-phosphorothioates.
Wilk A, et al.
Journal of the American Chemical Society, 122(10), 2149-2156 (2000)
Synthesis and thermal characterization of novel poly (tetramethylsilanthrylenesiloxane) and poly (tetramethylsilphenanthrylenesiloxane) derivatives.
Sato I, et al.
Polymer Bull., 59(5), 607-617 (2007)
G V S M Carrera et al.
Faraday discussions, 183, 429-444 (2015-09-22)
In this report, novel systems, based on highly abundant saccharides, D-mannose, D-glucose, β-cyclodextrin, alginic acid and mannitol, in combination with an organic superbase, tetramethylguanidine (TMG) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), are studied for carbon dioxide capture. With D-mannose and D-glucose, several ratios
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/237/769/028967ef-ca63-4f22-acc9-68f135a43b9a/640/028967ef-ca63-4f22-acc9-68f135a43b9a.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)



![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
