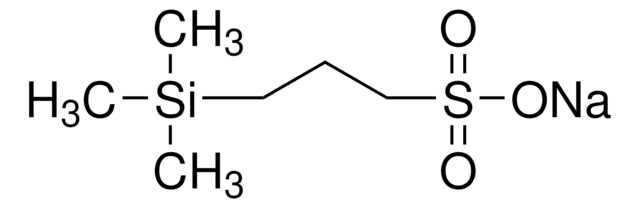
681334
2-(Trimethylsilyl)ethanesulfonyl chloride
Synonym(s):
2-Trimethylsilylethylsulfonyl chloride, SES-Cl
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3SiCH2CH2SO2Cl
CAS Number:
Molecular Weight:
200.76
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.4444
bp
146.8 °C/760 mmHg
density
1.059 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
C[Si](C)(C)CCS(Cl)(=O)=O
InChI
1S/C5H13ClO2SSi/c1-10(2,3)5-4-9(6,7)8/h4-5H2,1-3H3
InChI key
BLPMCIWDCRGIJV-UHFFFAOYSA-N
Related Categories
Application
Reactant involved in:
- Nucleophilic substitution for synthesis of nicotinamine and its analogs
- Regioselective metal-free oxidative cyclization of sulfonamides
- Annulation reactions
- Tin-free radical carbonylation of alkylsulfonyl derivatives
- Asymmetric aziridination
Reagent used for protection of an amino group as a SES-amide. The SES group can be conveniently removed by fluoride ion.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
70.0 °F - closed cup
Flash Point(C)
21.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-(Trimethylsilyl)ethanesulfonyl (or SES) group in amine protection and activation.
Patrice Ribière et al.
Chemical reviews, 106(6), 2249-2269 (2006-06-15)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1-[2-(Trimethylsilyl)ethoxycarbonyloxy]pyrrolidin-2,5-dione](/deepweb/assets/sigmaaldrich/product/structures/315/669/b4696f8e-7012-4d38-8354-dcfd174cc558/640/b4696f8e-7012-4d38-8354-dcfd174cc558.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)