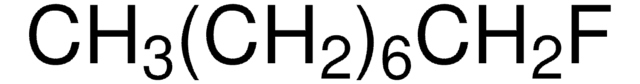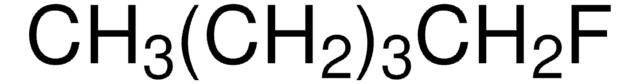538647
1-Fluorododecane
98%
Synonym(s):
n-Dodecyl fluoride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F(CH2)11CH3
CAS Number:
Molecular Weight:
188.33
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.42 (lit.)
density
0.807 g/mL at 25 °C (lit.)
functional group
alkyl halide
fluoro
SMILES string
CCCCCCCCCCCCF
InChI
1S/C12H25F/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-12H2,1H3
InChI key
YHYBNVZCQIDLSQ-UHFFFAOYSA-N
General description
1-Fluorododecane is a long chain 1-fluoroalkane. It can be synthesized from the reaction between 1-dodecanol, N,N-diethyl-α,α-difluoro-[3,5-bis(1H,1H,2H,2H-perfluorodecyl)benzyl]amine and heptane. 1-Fluorododecane undergoes reaction with dibromomethane and titanocene dichloride in the presence of triethyl aluminium (Et3Al) to afford a mixture of 1-chlorododecane and 1-bromododecane. 1-Fluorododecane can also be prepared from 1-hydroxydodecane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Aquatic Chronic 4
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
223.0 °F - closed cup
Flash Point(C)
106.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fluorination of alcohols and diols with a novel fluorous deoxy-fluorination reagent.
Furuya T, et al.
Journal of Fluorine Chemistry, 130(2), 348-353 (2009)
Halogen Exchange Reaction of Aliphatic Fluorine Compounds with Organic Halides as Halogen Source.
Mizukami Y, et al.
Organic Letters, 17(24), 5942-5945 (2015)
Regioselective Hydroxylation of C12?C15 Fatty Acids with Fluorinated Substituents by Cytochrome P450 BM3.
Chiang, Chih-Hsiang, et al.
Chemistry?A European Journal, 19(41), 13680-13691 (2013)
Vapor Pressures and Boiling Points of the 1-Fluoroalkanes, 1-Chloroalkanes, 1-Bromoalkanes, and 1-Iodoalkanes, C1to C20.
Li JCM and Rossini RD.
Journal of Chemical and Engineering Data, 6(2), 268-270 (1961)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service