All Photos(2)
About This Item
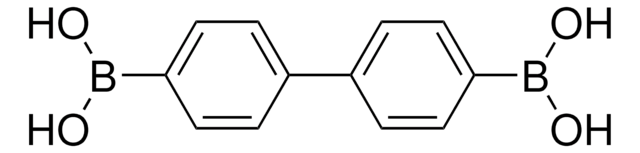
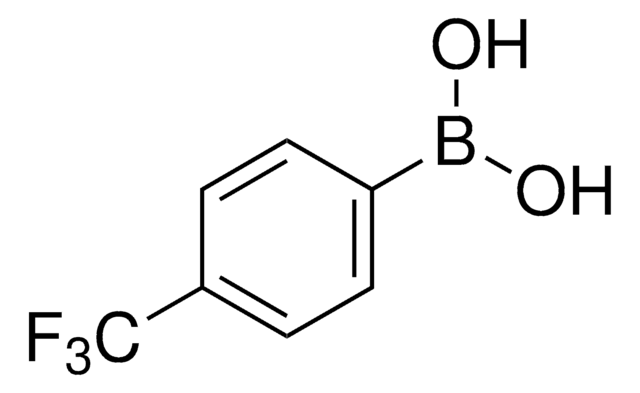
Linear Formula:
C6H5C6H4B(OH)2
CAS Number:
Molecular Weight:
198.03
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
232-245 °C (lit.)
SMILES string
OB(O)c1ccc(cc1)-c2ccccc2
InChI
1S/C12H11BO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9,14-15H
InChI key
XPEIJWZLPWNNOK-UHFFFAOYSA-N
Related Categories
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kazuhiko Tsukagoshi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 23(2), 227-230 (2007-02-14)
In our previous study, we proposed molecular recognition of mono- and disaccharides making use of the interaction between their diol groups and p-iodophenylboronic acid in capillary electrophoresis with a chemiluminescence detection system. Here, to extend our knowledge of molecular recognition
L J Kricka et al.
Journal of bioluminescence and chemiluminescence, 11(3), 137-147 (1996-05-01)
The enhancers 1,1'-biphenyl-4-yl boronic acid and 4-iodophenol act synergistically in the horseradish peroxidase-catalysed oxidation of luminol. This concentration-dependent effect reduces background, increases signal and hence improves signal/background for detection of peroxidase. The same type of synergistic effect was found when
Steven R Inglis et al.
Journal of medicinal chemistry, 52(19), 6097-6106 (2009-09-08)
Penicillin binding proteins (PBPs) catalyze steps in the biosynthesis of bacterial cell walls and are the targets for the beta-lactam antibiotics. Non-beta-lactam based antibiotics that target PBPs are of interest because bacteria have evolved resistance to the beta-lactam antibiotics. Boronic
Anna Minkkilä et al.
Journal of medicinal chemistry, 51(22), 7057-7060 (2008-11-06)
A series of commercial phenyl-, heteroaryl-, alkyl-, and alkenylboronic acids were evaluated for their FAAH and MGL inhibitory activities. The compounds were generally selective for FAAH, with IC50 in the nanomolar or low-micromolar range. Eight of these compounds inhibited MGL
Alessio Innocenti et al.
Bioorganic & medicinal chemistry letters, 19(10), 2642-2645 (2009-04-21)
Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalkylboronic acids was investigated. Aromatic, 4-phenylsubstituted- and 2-naphthylboronic acids were the best Can2 inhibitors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service