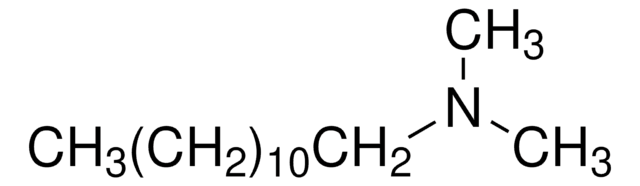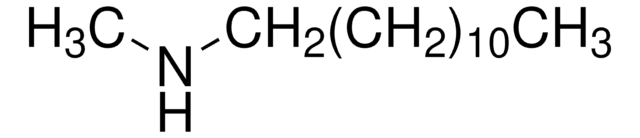464287
N,N-Dimethyldecylamine
98%
Synonym(s):
1-(Dimethylamino)decane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
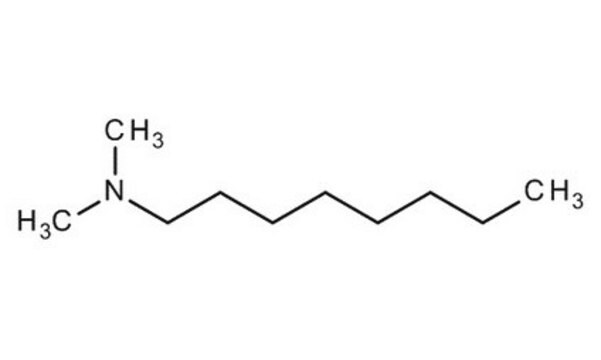
CH3(CH2)9N(CH3)2
CAS Number:
Molecular Weight:
185.35
Beilstein:
1738198
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.431 (lit.)
bp
234 °C (lit.)
density
0.778 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCCCCCCCCCN(C)C
InChI
1S/C12H27N/c1-4-5-6-7-8-9-10-11-12-13(2)3/h4-12H2,1-3H3
InChI key
YWWNNLPSZSEZNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
N,N-Dimethyldecylamine (DMDA) is a tertiary amine.
Application
N,N-Dimethyldecylamine (DMDA) may be used for the following studies:
- Pore expansion of the aminopropyl-functionalized ethane-bridged bifunctional periodic mesoporous organosilicas (APEPMOs).
- Preparation of stationary phase (named QA C10) having a quaternary ammonium embedded between a propyl and a decyl chain.
- Pore exapansion of monodisperse phenylene-bridged organosilica spheres.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liye Guan et al.
Biotechnology letters, 35(8), 1323-1330 (2013-04-18)
The aminopropyl-functionalized ethane-bridged bifunctional periodic mesoporous organosilicas (APEPMOs) were synthesized by the co-condensation of 1,2-bis (triethoxysilyl) ethane and 3-aminopropyltriethoxysilane in the presence of cationic surfactants octadecyltrimethylammonium chloride in basic medium. The pores of the APEPMOs were expanded with N,N-dimethyldecylamine and
Qiaoxia Liu et al.
Journal of separation science, 35(20), 2685-2692 (2012-09-26)
A stationary phase (named QA C10) with quaternary ammonium embedded between a propyl and a decyl chain was synthesized by immobilization of N,N-dimethyldecylamine on chloropropyl-silica surface. A set of representative neutral, basic, and acidic compounds was employed to evaluate its
Yongping Zhang et al.
Talanta, 81(3), 824-830 (2010-03-20)
Monodisperse phenylene-bridged organosilica spheres show great potential as chromatographic stationary phase. In this paper, the tunable particle size of monodisperse phenylene-bridged organosilica spheres were prepared by co-condensing different proportion of 1,4-bis(triethoxysilyl)benzene (1,4-BTEB) and tetraethylorthosilicate (TEOS), and then pore size was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service