446653
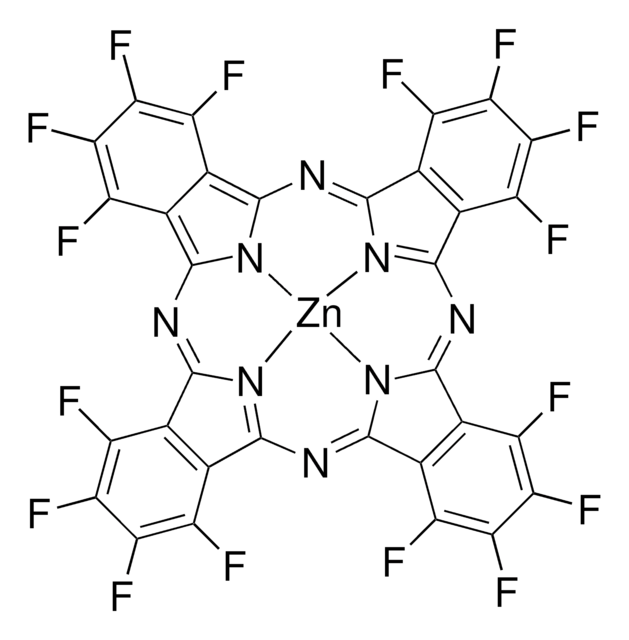
Copper(II) 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine
Dye content 80 %
Synonym(s):
F16CuPc
About This Item
Recommended Products
form
powder
Quality Level
composition
Dye content, 80%
mp
>300 °C (lit.)
λmax
689 nm
SMILES string
Fc1c(F)c(F)c2c3nc(nc4n5[Cu]n6c(n3)c7c(F)c(F)c(F)c(F)c7c6nc8nc(nc5c9c(F)c(F)c(F)c(F)c49)c%10c(F)c(F)c(F)c(F)c8%10)c2c1F
InChI
1S/C32F16N8.Cu/c33-9-1-2(10(34)18(42)17(9)41)26-49-25(1)53-27-3-4(12(36)20(44)19(43)11(3)35)29(50-27)55-31-7-8(16(40)24(48)23(47)15(7)39)32(52-31)56-30-6-5(28(51-30)54-26)13(37)21(45)22(46)14(6)38;/q-2;+2
InChI key
FJAOBQORBYMRNO-UHFFFAOYSA-N
Related Categories
General description
Application
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Flexible electronic circuits and displays based on organic active materials are future generations of products that may eventually enter mainstream electronics market.
Self-Assembled Nanodielectrics (SANDs) for Unconventional Electronics
Fabrication procedure of organic field effect transistor device using a soluble pentacene precursor.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11-hexacarbonitrile 95% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/151/558/c0e2c95f-5228-4864-a7a5-4b9765a19840/640/c0e2c95f-5228-4864-a7a5-4b9765a19840.png)