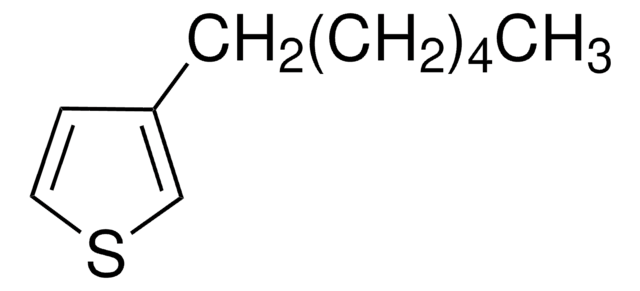445703
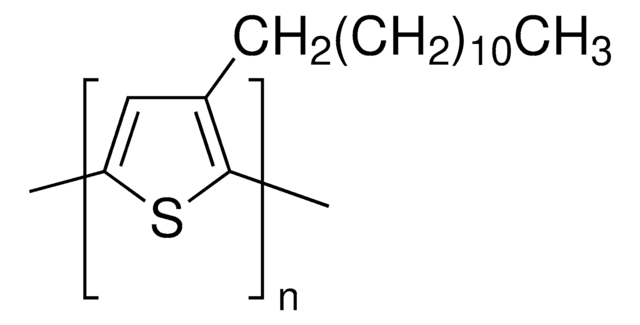
Poly(3-hexylthiophene-2,5-diyl)
regioregular
Synonym(s):
P3HT
About This Item
Recommended Products
mol wt
average Mw 50,000-100,000
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
conductivity
~103 S/cm (when doped with iodine)
mp
238 °C
238 °C
fluorescence
λex 443 nm; λem 568 nm in chloroform
Orbital energy
HOMO 5 eV
LUMO 3 eV
OPV Device Performance
ITO/NiO/P3HT/PC61BM/LiF/Al
ITO/PEDOT:PSS/P3HT:PC61BM (1:08)/Al
greener alternative category
semiconductor properties
P-type (mobility=1E-4-1E-1 cm2/V·s)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Features and Benefits
Good processibility, environmental stability and electroactivity.
Packaging
Legal Information
Rieke is a registered trademark of Rieke Metals, Inc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Flexible electronic circuits and displays based on organic active materials are future generations of products that may eventually enter mainstream electronics market.
Polymer-based Materials for Printed Electronics: Enabling High Efficiency Solar Power and Lighting
The union of distinct scientific disciplines is revealing the leading edge of Nanotechnology.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[6,6]-Phenyl C61 butyric acid methyl ester ≥99%](/deepweb/assets/sigmaaldrich/product/structures/359/221/d990c746-0960-4c69-bf76-fe09b193824d/640/d990c746-0960-4c69-bf76-fe09b193824d.png)