All Photos(2)
About This Item
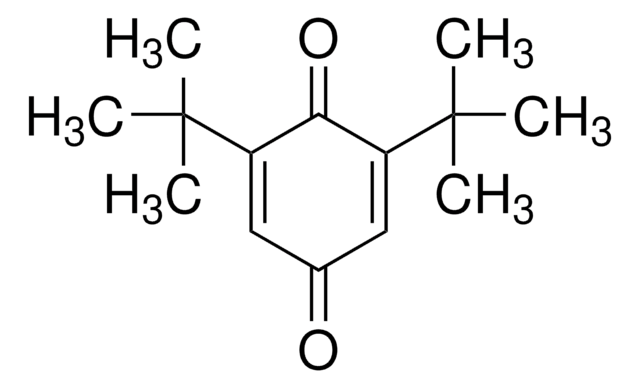
Linear Formula:
(CH3)3CC6H3(=O)2
CAS Number:
Molecular Weight:
164.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
54-58 °C (lit.)
functional group
ketone
SMILES string
CC(C)(C)C1=CC(=O)C=CC1=O
InChI
1S/C10H12O2/c1-10(2,3)8-6-7(11)4-5-9(8)12/h4-6H,1-3H3
InChI key
NCCTVAJNFXYWTM-UHFFFAOYSA-N
General description
2-tert-Butyl-1,4-benzoquinone (TBQ, TBBQ, tBQ, BuBQ, BQ , tert-butyl-p-quinone) is a 1,4-benzoquinone derivative. It is a major metabolite of the food additive, butylated hydroxyanisole (BHA). TBQ is reported to be strongly cytotoxic in human monocytic leukemia U937 cells. TBQ is an oxidation product of 2-tert-butylhydroquinone (TBHQ). Studies confirm that TBQ induces apoptosis and cell proliferation inhibition in chronic myelogenous leukemia (CML) cells. Its binding interactions with lysozyme has been examined and found to be intermediate between BHA and TBHQ. It has been reported to be synthesized by the titanium superoxide catalyzed oxidation of 2-tert-butylphenol using aq. 30% H2O2. TBQ is one of the main neoformed compounds from TBHQ decomposition in PLA-TBHQ film (Poly lactic acid).
Application
2-tert-Butyl-1,4-benzoquinone may be used in the synthesis of azatrioxa[8]circulene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-tert-butyl-1, 4-benzoquinone Induces Apoptosis in Chronic Myeloid Leukemia Cells Resistant to Imatinib via Inducing Caspase-Dependent Bcr-Abl Downregulation.
Shi X, et al.
Medicinal Chemistry, 4, 784-790 (2014)
R Kahl et al.
Toxicology, 59(2), 179-194 (1989-12-01)
The synthetic antioxidant butylated hydroxyanisole (BHA) stimulates superoxide formation in rat liver microsomes up to 10-fold. This stimulation is prevented by the monooxygenase inhibitor metyrapone and does not occur when NADH is consumed instead of NADPH indicating that metabolic activation
W H Kalus et al.
Environmental health perspectives, 102(1), 96-99 (1994-01-01)
We examined t-butylhydroquinone (t-BHQ) and t-butylquinone (t-BuQ), two of the major microsomal metabolites of the synthetic antioxidant butylated hydroxyanisole (BHA), for their ability to react with the xenobiotic arylamines aniline and N-methylaniline. A number of substances were isolated by thin-layer
Azatrioxa [8] circulenes: Planar Anti-Aromatic Cyclooctatetraenes.
Nielsen CB, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 19(12), 3898-3904 (2013)
P A Schilderman et al.
Carcinogenesis, 16(3), 507-512 (1995-03-01)
The food additive butylated hydroxyanisole (BHA) has been shown to induce gastrointestinal hyperplasia in rodents by an unknown mechanism. The relevance of this observation for human risk assessment is not clear. We therefore analysed the effect of BHA and its
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service