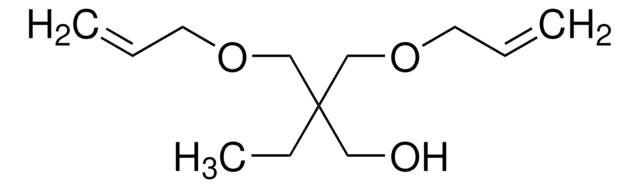
416134
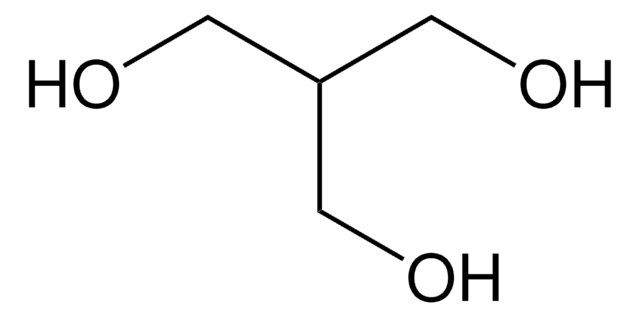
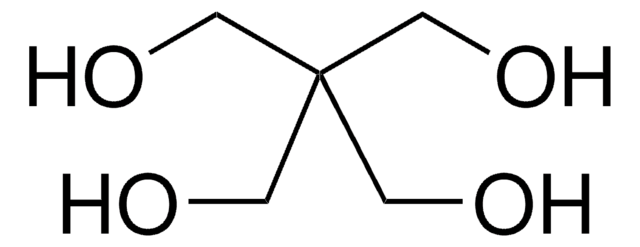
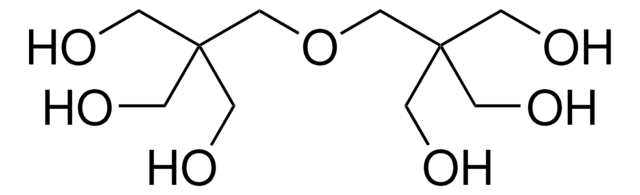
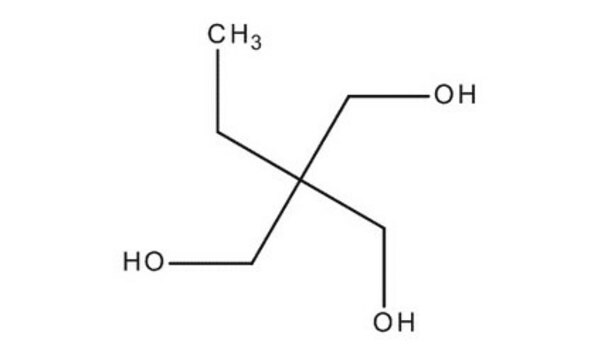
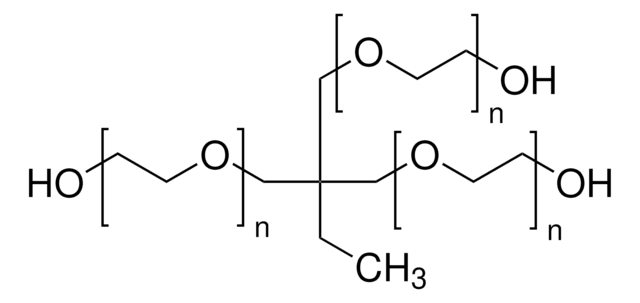
Di(trimethylolpropane)
97%
Synonym(s):
2,2′-Oxybis(methylene)bis(2-ethyl-1,3-propanediol)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O[CH2C(C2H5)(CH2OH)2]2
CAS Number:
Molecular Weight:
250.33
Beilstein:
1860782
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
bp
215 °C/4 mmHg (lit.)
mp
108-111 °C (lit.)
SMILES string
CCC(CO)(CO)COCC(CC)(CO)CO
InChI
1S/C12H26O5/c1-3-11(5-13,6-14)9-17-10-12(4-2,7-15)8-16/h13-16H,3-10H2,1-2H3
InChI key
WMYINDVYGQKYMI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Di(trimethylolpropane) is a monomeric unit with primary hydroxyl groups, that can be used as a tetrafunctional core molecule.
Application
Di(trimethylolpropane) can be used for the synthesis of lipase catalyzed hyperbranched polymers. It can also be used in the formation of biodegradable polymers for drug delivery applications.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, characterization and in vitro degradation of 3D-microstructured poly (varepsilon-caprolactone) resins
Theiler S, et al.
Polym. Chem., 1(8), 1215-1225 (2010)
Rheological properties of concentrated solutions of aliphatic hyperbranched polyesters
Vukovic J, et al.
Macromolecular Chemistry and Physics, 208(21), 2321-2330 (2007)
Biodegradable cross-linked poly (trimethylene carbonate) networks for implant applications: Synthesis and properties
Yang L, et al.
Polymer, 54(11), 2668-2675 (2013)
Ankur S Kulshrestha et al.
Biomacromolecules, 8(6), 1794-1801 (2007-05-05)
Lipase-catalyzed terpolymerizations were performed with the monomers trimethylolpropane (B3), 1,8-octanediol (B2), and adipic acid (A2). Polymerizations were performed in bulk, at 70 degrees C, for 42 h, using immobilized lipase B from Candida antartica (Novozyme-435) as a catalyst. To determine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service