402516
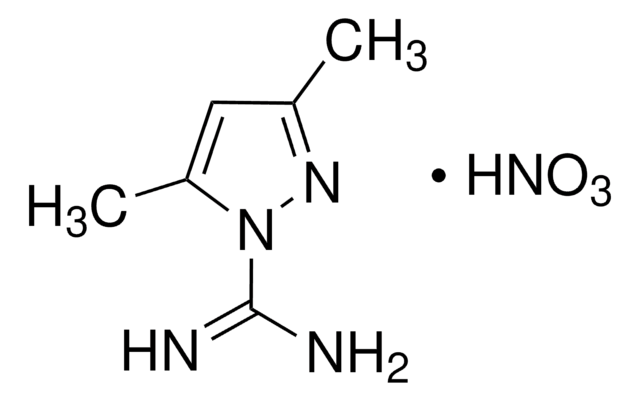
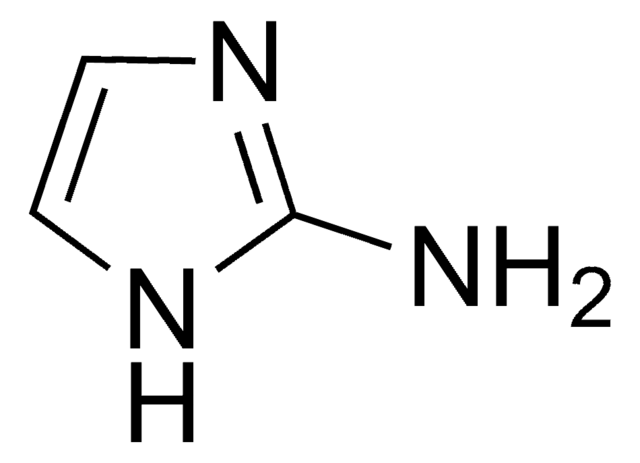
1H-Pyrazole-1-carboxamidine hydrochloride
99%
Synonym(s):
1-Amidinopyrazole hydrochloride, Praxadine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H6N4 · HCl
CAS Number:
Molecular Weight:
146.58
Beilstein:
5448758
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
167-170 °C (lit.)
functional group
amine
SMILES string
Cl[H].NC(=N)n1cccn1
InChI
1S/C4H6N4.ClH/c5-4(6)8-3-1-2-7-8;/h1-3H,(H3,5,6);1H
InChI key
RBZRMBCLZMEYEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1H-Pyrazole-1-carboxamidine hydrochloride, a pyrazole derivative, is a heterocyclic compound. It is widely used in drug syntheses studies. Pyrazole ring forms the main core of various nonsteroidal anti-inflammatory drugs (NSAIDs) and antihypertensive drugs.
Application
1H-Pyrazole-1-carboxamidine hydrochloride may be used in the following studies:
- Preparation of guanidylated hollow fiber membranes.
- Guanylation of amines and in peptide synthesis.
- Synthesis of bis-guanidinium-cholesterol derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Sens. 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hironori Izawa et al.
Biomolecules, 9(7) (2019-07-10)
In order to synthesize a promising material for developing a novel peptide/protein delivery system, guanidinylation of chitooligosaccharides with 1-amidinopyrazole hydrochloride was investigated herein. The production of guanidinylated chitooligosaccharides was demonstrated by infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and elemental
1H-Pyrazole-1-carboxamidine hydrochloride an attractive reagent for guanylation of amines and its application to peptide synthesis.
Bernatowicz MS, et al.
The Journal of Organic Chemistry, 57(8), 2497-2502 (1992)
Guanidinium-cholesterol cationic lipids: efficient vectors for the transfection of eukaryotic cells.
J P Vigneron et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(18), 9682-9686 (1996-09-03)
Two cationic lipids, bis-guanidinium-spermidine-cholesterol (BGSC) and bis-guanidinium-trencholesterol (BGTC)-cholesterol derivatives bearing two guanidinium groups-have been synthesized and tested as artificial vectors for gene transfer. They combine the membrane compatible features of the cholesterol subunit and the favorable structural and high pKa
Xiao Zhang et al.
ACS applied materials & interfaces, 12(14), 16088-16096 (2020-03-17)
Supramolecular hydrogels have great potential as biomaterials for tissue engineering applications or vehicles for delivering therapeutic agents. Herein, a self-healing and pro-osteogenic hydrogel system is developed based on the self-assembly of laponite nanosheets and guanidinylated chitosan, where laponite works as
Ekaterina K Ogurtsova et al.
Natural product communications, 10(7), 1171-1173 (2015-09-29)
The guanidine alkaloids, dihydropulchranin A (2), prepared from pulchranin A from the sponge Monanchora pulchra, and hexadecylguanidine (3), a synthetic analog of pulchranins, were studied for their TRPV channel-regulating activities. Compound 2 was active as an inhibitor of rTRPV1 and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service