All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C17H22O
CAS Number:
Molecular Weight:
242.36
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
powder
mp
128-130 °C (lit.)
SMILES string
Cc1ccc(O)c(c1)C23C[C@H]4C[C@H](C[C@H](C4)C2)C3
InChI
1S/C17H22O/c1-11-2-3-16(18)15(4-11)17-8-12-5-13(9-17)7-14(6-12)10-17/h2-4,12-14,18H,5-10H2,1H3/t12-,13+,14-,17-
InChI key
XHLJIHBDAJFXBE-ZZNDEYBLSA-N
Related Categories
General description
2-(1-Adamantyl)-4-methylphenol is a 2-adamantylphenol derivative. It has been synthesized by the adamantylation of p-cresol with 1-adamantanol. The structure has been confirmed by 1H and 13C NMR.
Application
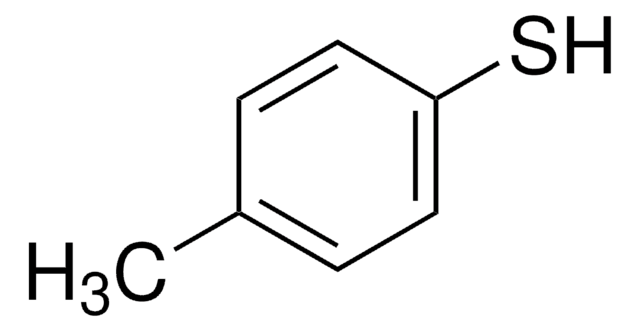
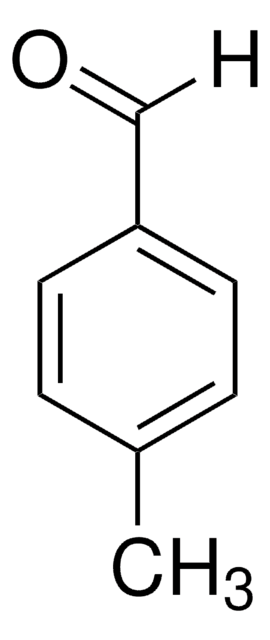
2-(1-Adamantyl)-4-methylphenol is a suitable starting reagent used in the synthesis of 2-(1-adamantyl)-4-methylthiophenol. It may also be used in the synthesis of 3-(1-adamantyl)-2-hydroxy-5-methylbenzaldehyde by reacting with urotropin. It may be used in the synthesis of adamantyl-substituted chromium(III) complex, a catalyst for enantioselective hetero-Diels-Alder reactions.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Reactions of 2-hydroxymethylphenols with Lawesson's reagent.
Osyanin VA, et al
Chemistry of Heterocyclic Compounds, 47(7), 901-905 (2011)
Synthesis of 2, 4-disubstituted thiophenols and solid state structures of thiocarbamate precursors.
Flores-Figueroa A, et al
Journal of the Brazilian Chemical Society, 16.3A, 397-403 (2005)
Highly Enantio-and Diastereoselective Hetero-Diels?Alder Reactions Catalyzed by New Chiral Tridentate Chromium (iii) Catalysts.
Dossetter AG, et al.
Angewandte Chemie (International Edition in English), 38(16), 2398-2400 (1999)
Nan Wang et al.
Beilstein journal of organic chemistry, 8, 227-233 (2012-03-17)
A clean process has been developed for the synthesis of 2-adamantylphenol derivatives through adamantylation of substituted phenols with adamantanols catalyzed by commercially available and recyclable ion-exchange sulfonic acid resin in acetic acid. The sole byproduct of the adamantylation reaction, namely
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service