380237
Tebbe reagent solution
0.5 M in toluene
Synonym(s):
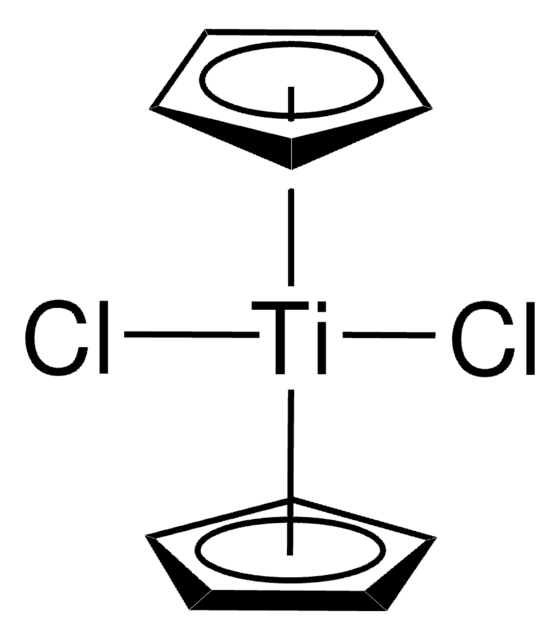
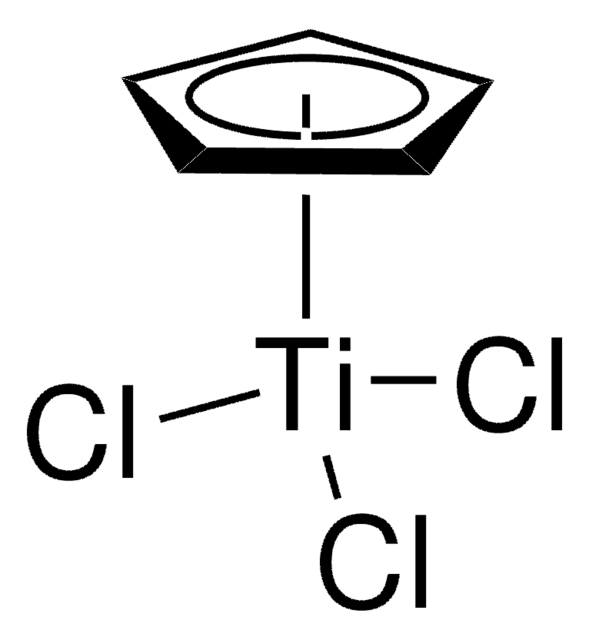
Bis(cyclopentadienyl)-μ-chloro(dimethylaluminum)-μ-methylenetitanium
About This Item
Recommended Products
form
liquid
Quality Level
reaction suitability
reaction type: C-C Bond Formation
concentration
0.5 M in toluene
density
0.927 g/mL at 25 °C
SMILES string
[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2.C[Al](C)C[Ti]Cl
InChI
1S/2C5H5.2CH3.CH2.Al.ClH.Ti/c2*1-2-4-5-3-1;;;;;;/h2*1-5H;2*1H3;1H2;;1H;/q;;;;;;;+1/p-1
InChI key
QEJAQNUJXFLWSP-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- For the conversion of carbonyl groups of chlorophyll derivatives into the corresponding exo-methylene (or vinylidene) groups.
- In the synthesis of β-C-glycosides from 3-OH glycol esters.
- As a versatile methylenation reagent for the conversion of ketones and aldehydes to olefins. It offers facile reaction with hindered ketones and allows the conversion of esters to vinyl ethers.
- To olefinate aldehydes.
- To methylenate a chiral polyhydroxyketone with high diasteroselctivity.
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 2 - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
39.2 °F - closed cup
Flash Point(C)
4 °C - closed cup
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service