All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H4OS2
CAS Number:
Molecular Weight:
168.24
Beilstein:
119513
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
74-77 °C (lit.)
solubility
toluene: soluble 2.5%, clear, yellow
functional group
disulfide
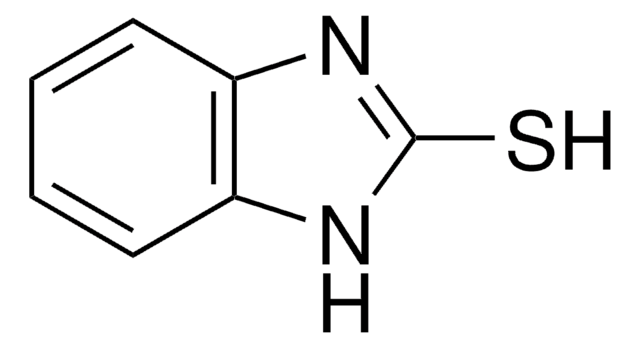
SMILES string
O=C1SSc2ccccc12
InChI
1S/C7H4OS2/c8-7-5-3-1-2-4-6(5)9-10-7/h1-4H
InChI key
GZTYTTPPCAXUHB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3H-1,2-Benzodithiol-3-one is a heterocyclic building block.
3H-1,2-Benzodithiol-3-one is reported to react with triphenylphosphine to afford dithiosalicylide.
Application
3H-1,2-Benzodithiol-3-one (1,2-benzodithiol-3-one) may be used as sulfur-transferring agent for the transformation of H-phosphonothioate and H-phosphonate diesters into the corresponding phosphorodi- and phosphoromonothioates.
3H-1,2-Benzodithiol-3-one may be employed as sulfurizing reagent in the preparation of phosphorothioate-containing oligodeoxyribonucleotides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Q Xu et al.
Nucleic acids research, 24(9), 1602-1607 (1996-05-01)
Previous methods for the preparation of phosphorothioate-containing oligodeoxyribonucleotides rely on the reaction of phosphite triesters with sulfurizing reagents such as tetraethylthiuram disulfide (TETD) and 3H-1,2-benzodithiol-3-one 1,1-dioxide (Beaucage reagent). However, these and other sulfurizing reagents suffer from several disadvantages, and there
Nucleoside H-phosphonates. 13. Studies on 3H-1, 2-benzodithiol-3-one derivatives as sulfurizing reagents for H-phosphonate and H-phosphonothioate diesters.
Stawinski J and Thelin M.
The Journal of Organic Chemistry, 56(17), 5169-5175 (1991)
Novel syntheses of dithiosalicylide.
Mitra K and Gates KS.
Tetrahedron Letters, 36(9), 1391-1394 (1995)
Santhosh Sivaramakrishnan et al.
Bioorganic & medicinal chemistry letters, 18(10), 3076-3080 (2007-12-11)
Though less potent than the parent natural product leinamycin, S-deoxyleinamycin displays activity against human cancer cell lines that is comparable to many clinically used agents. The results reported here suggest that the 1,2-dithiolan-3-one heterocycle found in S-deoxyleinamycin reacts with thiols
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service