All Photos(1)
About This Item
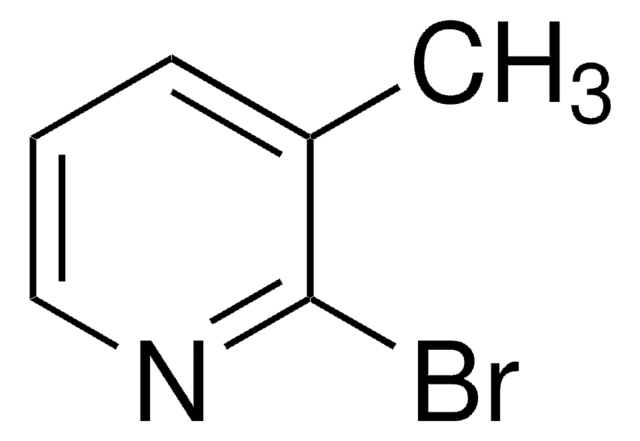
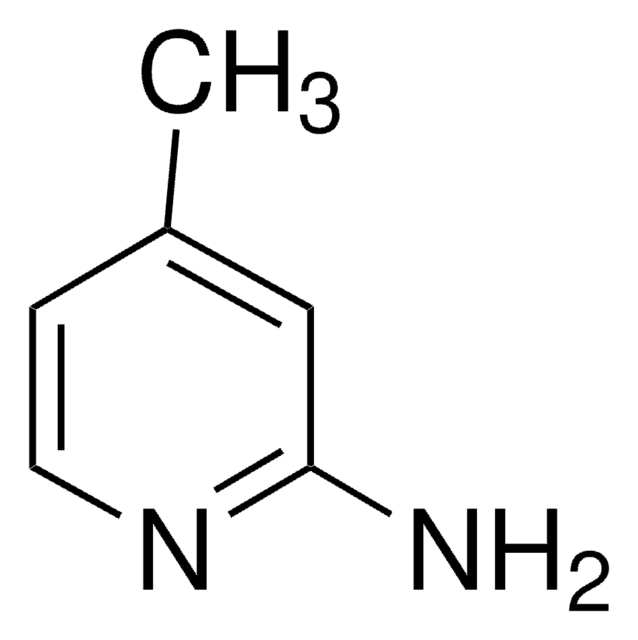
Empirical Formula (Hill Notation):
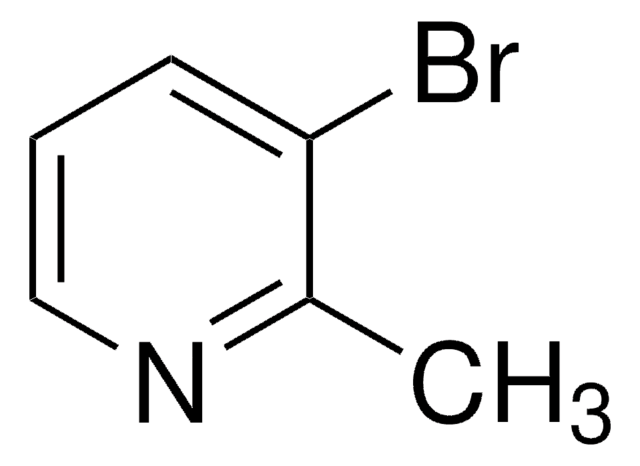
C6H6BrN
CAS Number:
Molecular Weight:
172.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.561 (lit.)
bp
87 °C/10 mmHg (lit.)
density
1.545 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Cc1ccnc(Br)c1
InChI
1S/C6H6BrN/c1-5-2-3-8-6(7)4-5/h2-4H,1H3
InChI key
LSZMVESSGLHDJE-UHFFFAOYSA-N
Application
2-Bromo-4-methylpyridine may be used:
- in the total synthesis of ocular age pigment A2-E (2 equiv of retinal (vitamin A) and 1 equiv of ethanolamine)
- in the preparation of methoxy-2-(2-pyridyl)indoles
- in the preparation of 2-(2′,4′-difluorophenyl)-4-methylpyridine, via a Suzuki coupling reaction with 2,4-difluorophenylboronic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Pd (0)-Catalyzed cross-coupling reactions of 2-indolylzinc halides. A convenient route to indolo [2, 3-a] quinolizidines.
Amat M, et al.
ARKIVOC (Gainesville, FL, United States), 73, 82-82 (2002)
Blue Emitting Cationic Iridium Complexes Containing Two Substituted 2-Phenylpyridine and One 2, 2'-Biimidazole for Solution-Processed Organic Light-Emitting Diodes (OLEDs).
Yun S-J, et al.
Bull. Korean Chem. Soc., 33(11), 3645-3650 (2012)
Total synthesis of the ocular age pigment A2-E: a convergent pathway.
Ren R X-F, et al.
Journal of the American Chemical Society, 119(15), 3619-3620 (1997)
Hector H Huang et al.
Nature communications, 11(1), 1931-1931 (2020-04-24)
Enhancing the efficacy of proteasome inhibitors (PI) is a central goal in myeloma therapy. We proposed that signaling-level responses after PI may reveal new mechanisms of action that can be therapeutically exploited. Unbiased phosphoproteomics after treatment with the PI carfilzomib surprisingly demonstrates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service