301787
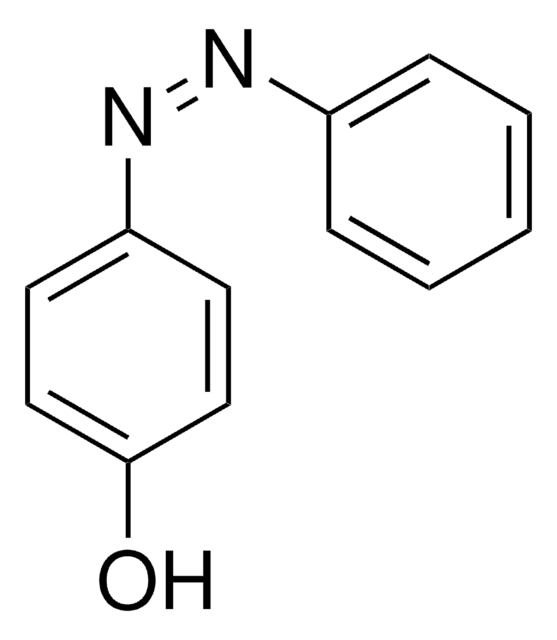
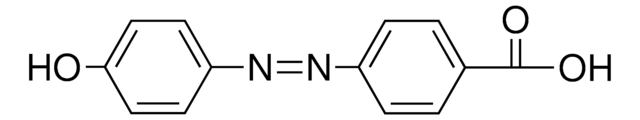
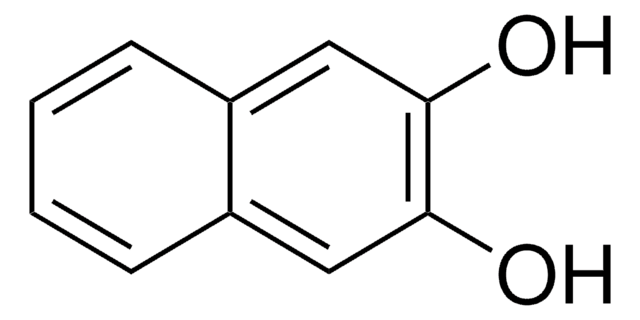
2,2′-Dihydroxyazobenzene
97%
Synonym(s):
2,2′-Azodiphenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4N=NC6H4OH
CAS Number:
Molecular Weight:
214.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
173-175 °C (lit.)
SMILES string
Oc1ccccc1\N=N\c2ccccc2O
InChI
1S/C12H10N2O2/c15-11-7-3-1-5-9(11)13-14-10-6-2-4-8-12(10)16/h1-8,15-16H/b14-13+
InChI key
JFEVWPNAOCPRHQ-BUHFOSPRSA-N
Related Categories
General description
2,2′-Dihydroxyazobenzene is a small molecule inhibitor of ADP ribosyl cyclase and it attenuates angiotensin (Ang) II-induced hypertrophic responses. 2,2′-Dihydroxyazobenzene is formed during the oxidation of diclofenac by myeloperoxidase/hydrogen peroxide.
Application
2,2′-Dihydroxyazobenzene was used as complexing reagent in determination of aluminum in human serum by ion-pair reversed-phase partition HPLC.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Kaneko et al.
Analytical chemistry, 63(20), 2219-2222 (1991-10-15)
An ion-pair reversed-phase partition high-performance liquid chromatography-spectrophotometric method is described for the determination of aluminum in human serum, based on its complexation with 2,2'-dihydroxyazobenzene. The chelate is separated on C18-bonded silica packing by using an aqueous methanol mobile phase containing
G Miyamoto et al.
Chemical research in toxicology, 10(4), 414-419 (1997-04-01)
Diclofenac is associated with a low, but significant, incidence of hepatotoxicity and bone marrow toxicity. It has been suggested that this could be due to a reactive acyl glucuronide. An alternative hypothesis is that an oxidative reactive metabolite could be
Dylan I Mori et al.
Advanced materials interfaces, 7(15) (2021-02-13)
Strategies to engineer surfaces that can enable the selective inhibition of bacterial pathogens while preserving beneficial microbes can serve as tools to precisely edit the microbiome. In the oral microbiome, this selectivity is crucial in preventing the proliferation of cariogenic
Rukhsana Gul et al.
Cardiovascular research, 81(3), 582-591 (2008-08-23)
Here, we report the discovery of a small molecule inhibitor, 2,2'-dihydroxyazobenzene (DAB), of ADP ribosyl cyclase (ADPR-cyclase) and showed that this inhibitor attenuated angiotensin (Ang) II-induced hypertrophic responses. and results The intracellular concentration of free Ca(2+) [Ca(2+)](i) in adult rat
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service