234982
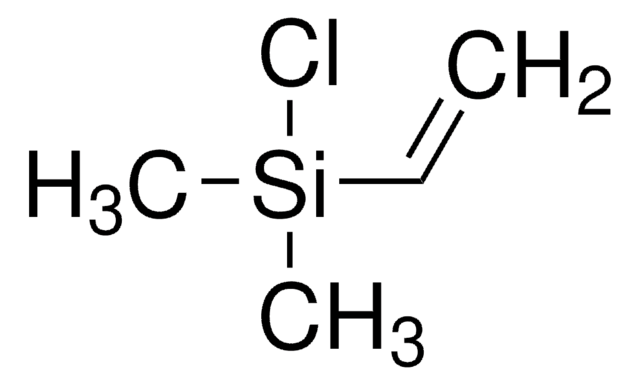
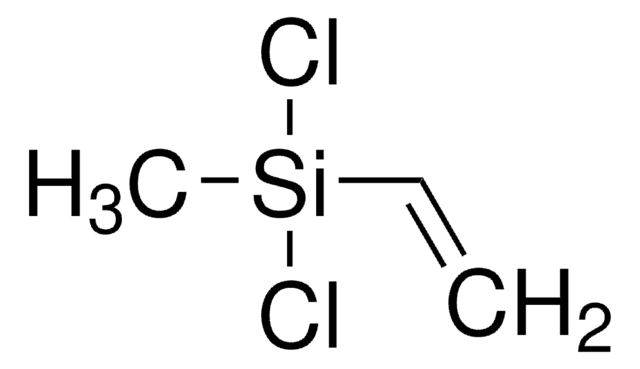
Allyl(chloro)dimethylsilane
97%
Synonym(s):
ADMCS, Allyldimethylchlorosilane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=CHCH2Si(CH3)2Cl
CAS Number:
Molecular Weight:
134.68
Beilstein:
1737978
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.427 (lit.)
bp
111-113 °C (lit.)
density
0.891 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(Cl)CC=C
InChI
1S/C5H11ClSi/c1-4-5-7(2,3)6/h4H,1,5H2,2-3H3
InChI key
KMVZWUQHMJAWSY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Allyl(chloro)dimethylsilane has been used in:
- the synthesis of cyclo-1,1′,4,4′-bis(1,1,3,3-tetramethyl-1,3-disiloxanediyl)dibenzene
- silylation of silicic acid
- the preparation of phenylenebis(silanediyl triflates), useful synthons for organosilicon polymers
- O-silylation of phenyl sulfones
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
42.8 °F - closed cup
Flash Point(C)
6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Helvetica Chimica Acta, 77, 972-972 (1994)
Oligo-and polysiloxanes.
Abe Y and Gunji T.
Progress in Polymer Science, 29(3), 149-182 (2004)
Journal of the American Chemical Society, 116, 5657-5657 (1994)
Structure of cyclo-1, 1′, 4, 4′-bis (1, 1, 3, 3-tetramethyl-1,3-disiloxanediyl) dibenzene.
Abboud KA, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 49(10), 1845-1848 (1993)
Katarzyna Mituła et al.
Polymers, 12(5) (2020-05-10)
The scientific reports on polyhedral oligomeric silsesquioxanes are mostly focused on the formation of completely condensed T8 cubic type structures and recently so-called double-decker derivatives. Herein, we report on efficient synthetic routes leading to trifunctionalized, open-cage silsesquioxanes with alkenyl groups
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service