178314
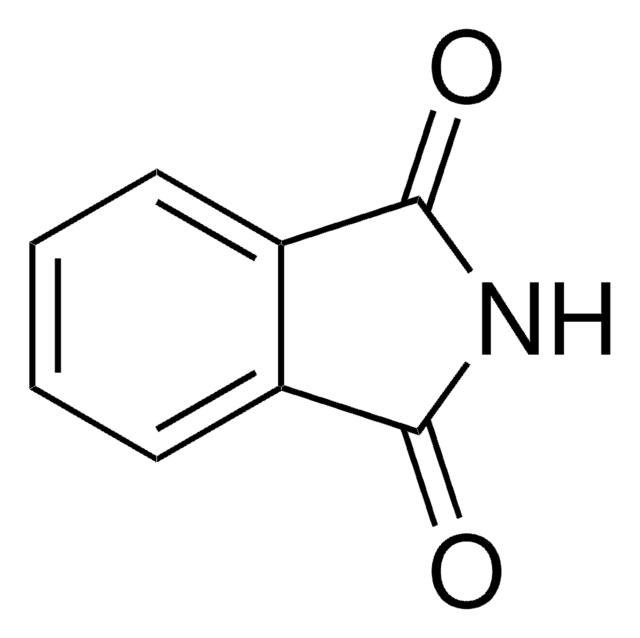
N-Aminophthalimide
technical grade, 90%
Synonym(s):
N,N-Phthaloylhydrazine, unsym.
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6N2O2
CAS Number:
Molecular Weight:
162.15
Beilstein:
383756
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
powder
mp
200-202 °C (lit.)
functional group
imide
SMILES string
NN1C(=O)c2ccccc2C1=O
InChI
1S/C8H6N2O2/c9-10-7(11)5-3-1-2-4-6(5)8(10)12/h1-4H,9H2
InChI key
KSILMCDYDAKOJD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
N-Aminophthalimide was employed in the aziridination of chiral N-enoyl sultams. It was also used in the synthesis of n-phthalimidoaziridines.
Other Notes
Remainder phthalhydrazide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
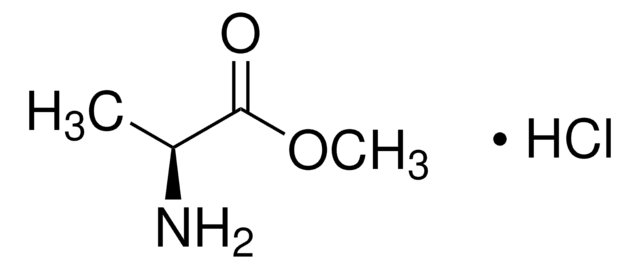
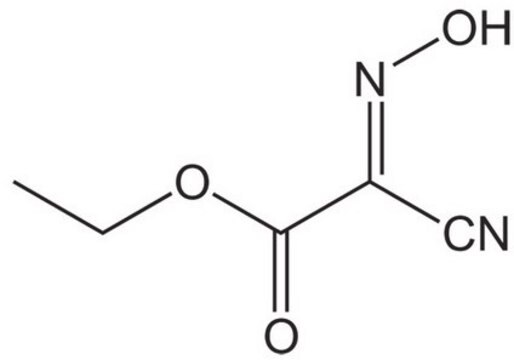
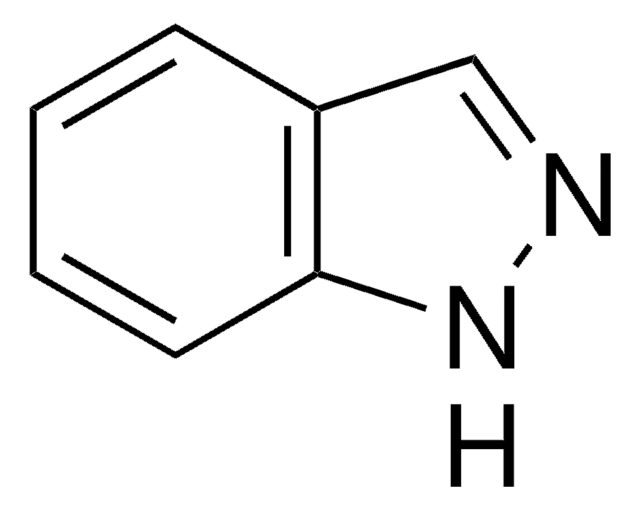
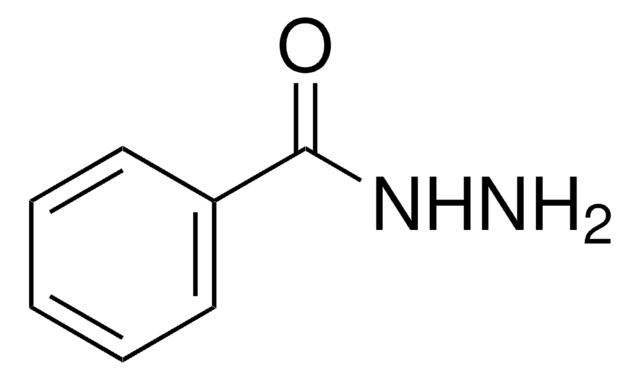
Customers Also Viewed
Journal of the Chemical Society. Chemical Communications, 1074-1074 (1993)
Kung-Shou Yang et al.
Organic letters, 4(7), 1107-1109 (2002-04-02)
[reaction: see text] Reaction of various N-enoyl oxazolidinones 5a-f with N-aminophthalimide and lead tetraacetate in the presence of camphor-derived chiral ligands provides the desired N-phthalimidoaziridines 6a-f in good to high enantiomeric excess (67-95% ee) at 0 degrees C within 15
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service