111279
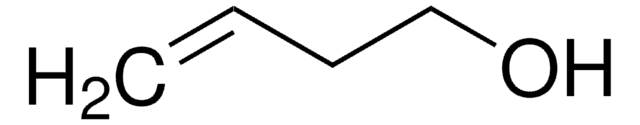
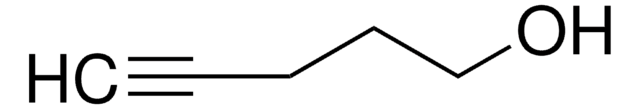
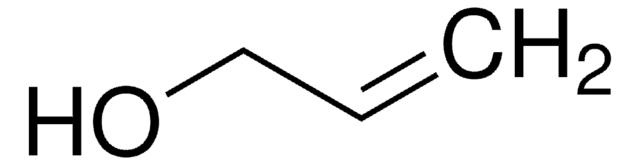
4-Penten-1-ol
99%
Synonym(s):
2-Allylethyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CH(CH2)3OH
CAS Number:
Molecular Weight:
86.13
Beilstein:
1560163
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39020310
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.429 (lit.)
bp
134-137 °C (lit.)
density
0.834 g/mL at 25 °C (lit.)
functional group
allyl
hydroxyl
SMILES string
OCCCC=C
InChI
1S/C5H10O/c1-2-3-4-5-6/h2,6H,1,3-5H2
InChI key
LQAVWYMTUMSFBE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-penten-1-ol forms ester bond at the C terminus of the linear peptide in solution with HATU as coupling agent.
Application
4-Penten-1-ol can be used as a reactant to prepare sulfamate ester by reacting with chlorosulfonyl isocycanate (142662).The derived ester undergoes an enantioselective intramolecular azridination reaction in the presence of Cu catalyst. 4-Penten-1-ol can also be used to study the epoxidation of olefins with oxo-diperoxo tungstate(VI) complex as catalyst and bicarbonate as co-catalyst.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective Intramolecular Copper-Catalyzed Aziridination of Sulfamates
Audrey Esteoule.et al.
Synthesis, 1251-1251 (2007)
Highly efficient epoxidation method of olefins with hydrogen peroxide as terminal oxidant, bicarbonate as a co-catalyst and oxodiperoxo molybdenum(VI) complex as catalyst.
Maiti SK, et al.
New. J. Chem., 30(3), 479-489 (2006)
Stefania Terracciano et al.
Bioorganic & medicinal chemistry, 16(13), 6580-6588 (2008-05-30)
In the recent years, we focused our attention on the cyclodepsipeptide Jaspamide 1, an interesting marine metabolite, possessing a potent inhibitory activity against breast and prostate cancer, as a consequence of its ability to disrupt actin cytoskeleton dynamics. Although its
Marina D Rvovic et al.
Journal of molecular modeling, 17(6), 1251-1257 (2010-08-17)
The mechanism of phenylselenoetherification of pent-4-en-1-ol using some bases (pyridine, triethylamine, quinoline, 2,2'-bipyridine) as catalyst was examined through studies of kinetics of the cyclization, by UV-VIS spectrophotometry. It was demonstrated that the intramolecular cyclization is facilitated in the presence of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service