P9120
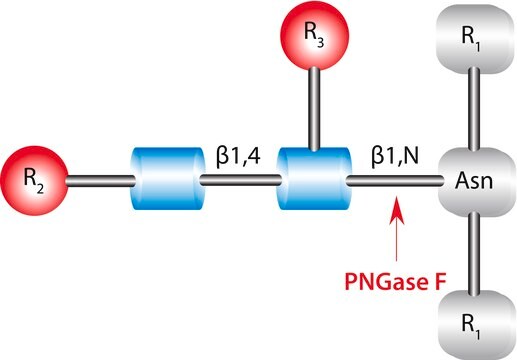
PNGase F from Elizabethkingia meningoseptica
recombinant, expressed in E. coli, set of 100 units nanomolar unit
Synonym(s):
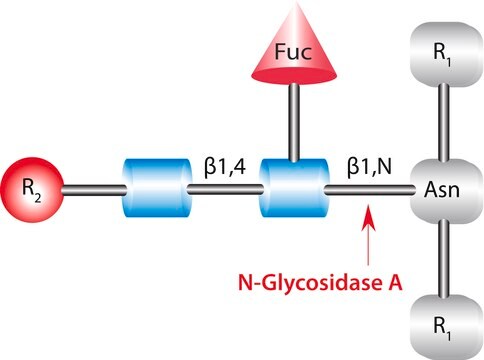
N-Glycanase®, N-Glycosidase F, PNGase F from Chryseobacterium meningosepticum, PNGase F from Flavobacterium meningosepticum, Peptide N-glycosidase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(N-linked)
specific activity
≥10 units/mg protein
mol wt
36 kDa
packaging
set of 100 units nanomolar unit
shipped in
wet ice
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
- of recombinant soybean agglutinin (rSBA) in Nicotiana benthamiana (NbrSBA) and Solanum tuberosum (StrSBA)
- of frontal cortical lysate to verify the glycosylation profile of β-secretase (BACE proteins)
- of cell lysate for evaluating the siRNA silencing of cellular prion protein (PrPc) post transfection
Biochem/physiol Actions
Packaging
Unit Definition
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Repr. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
N-Linked Glycan Strategies. Use of the endoglycosidic enzyme PNGase F (N-Glycosidase F) is the most effective method of removing virtually all N-linked oligosaccharides from glycoproteins.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service