F3879
Fibrinogen from human plasma
50-70% protein (≥80% of protein is clottable)
Synonym(s):
Factor I
About This Item
Recommended Products
biological source
human plasma
Quality Level
form
powder
quality
50-70% protein (≥80% of protein is clottable)
mol wt
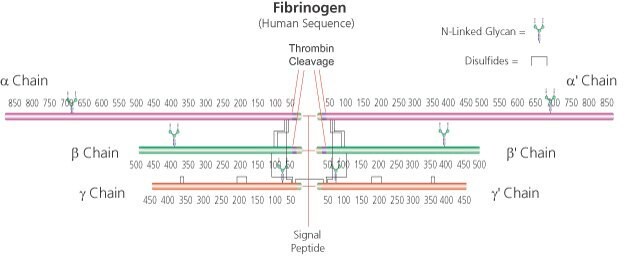
α-chain 63.5 kDa
β-chain 56 kDa
γ chain 47 kDa (about 4% carbohydrate content)
soluble dimer 340 kDa
concentration
50-70% protein (biuret)
technique(s)
ELISA: suitable
solubility
0.9% NaCl: soluble 10 mg/mL
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
Fibrinogen was also used in the development of a fibrinogen-specific sandwich enzyme-linked immunosorbent assay microarray assay for distinguishing between blood plasma and serum samples.
Fibrinogen from human plasma has been used-
- for the production of fibrin hydrogels
- for the preparation of fibrin-MSCs (mesenchymal stem cells)-cartilage constructs
- for analyzing the protein repellent properties of PFDA-co-DEGDME (diethyleneglycol dimethyl ether) surface using Quartz crystal microbalance (QCM)
Biochem/physiol Actions
Specifications
Physical form
Reconstitution
Analysis Note
Disclaimer
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We will explore the technological advances that have contributed toward the progress of 3DP of tissue engineering scaffolds, current materials used to create 3DP scaffolds, and the challenges that remain.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service