E2264
Endoglycosidase F3 from Elizabethkingia miricola
recombinant, expressed in E. coli, 30 U/mg
Synonym(s):
Elizabethkingia miricola, Endo-β-N-acetylglucosaminidase F3, Endoglycosidase F3 from Elizabethkingia (Chryseobacterium/Flavobacterium) meningosepticum
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(N-linked)
form
solution
specific activity
30 U/mg
mol wt
32 kDa
shipped in
wet ice
storage temp.
2-8°C
Related Categories
Application
Endoglycosidase F3 from Elizabethkingia miricola has been used to analyze core fucosylation and tryptic digests of serum proteins.
Biochem/physiol Actions
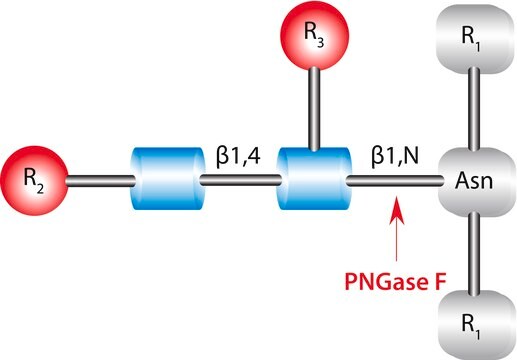
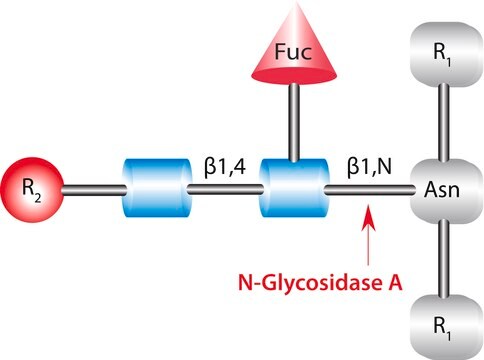
Endoglycosidase F3 belongs to the glycoside hydrolase family 18 (GH18). It has hydrolytic activity. Endoglycosidase F3 glycosylates α-1,6-fucosylated GlcNAc derivative to give natural, core fucosylated complex-type N-glycopeptides.
Cleaves asparagine-linked biantennary and triantennary complex, oligosaccharides depending on the state of core fucosylation and peptide linkage.
Packaging
Supplied with 5× Reaction Buffer, 250 mM sodium acetate, pH 4.5
Unit Definition
One unit will release N-linked oligosaccharides from 1 μmole of denatured porcine fibrinogen in 1 minute at 37 °C, pH 4.5.
Physical form
Aseptically filled solution in 20 mM Tris-HCl, pH 7.5
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Characterization of novel endo-beta-N-acetylglucosaminidases from Sphingobacterium species, Beauveria bassiana and Cordyceps militaris that specifically hydrolyze fucose-containing oligosaccharides and human IgG
Huang Y, et al.
Scientific reports, 8(1), 246-246 (2018)
Chemical Biology of Glycoproteins (2017)
Advances in Carbohydrate Chemistry (2016)
Quantitative analysis of core fucosylation of serum proteins in liver diseases by LC-MS-MRM
Ma J, et al.
Journal of proteomics, 189, 67-74 (2018)
Roger S Zou et al.
Aging, 3(10), 968-984 (2011-10-13)
A distinct conformational transition from the α-helix-rich cellular prion protein (PrPC) into its β-sheet-rich pathological isoform (PrPSc) is the hallmark of prion diseases, a group of fatal transmissible encephalopathies that includes spontaneous and acquired forms. Recently, a PrPSc-like intermediate form
Articles
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service