36798
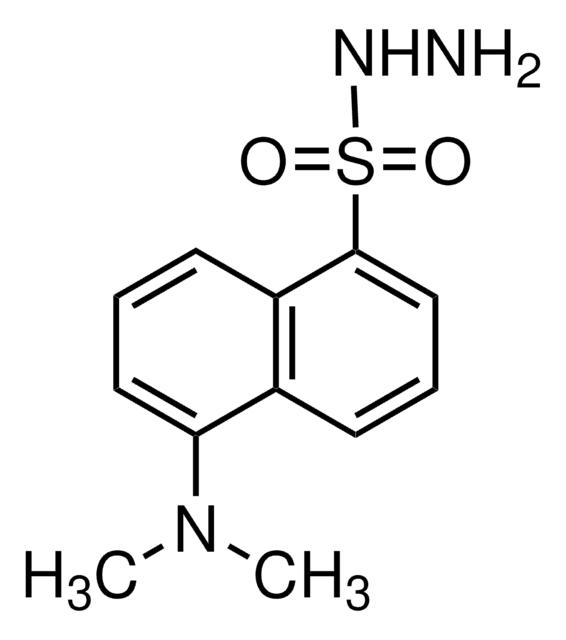
7-(Diethylamino)coumarin-3-carbohydrazide
BioReagent, suitable for fluorescence, ≥95% (HPCE)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H17N3O3
CAS Number:
Molecular Weight:
275.30
Beilstein:
5574454
MDL number:
UNSPSC Code:
12352125
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
product line
BioReagent
Quality Level
Assay
≥95% (HPCE)
solubility
DMF: soluble
acetonitrile: soluble
chloroform: soluble
methanol: soluble
fluorescence
λex 450 nm; λem 468 nm in methanol
suitability
suitable for fluorescence
SMILES string
CCN(CC)c1ccc2C=C(C(=O)NN)C(=O)Oc2c1
InChI
1S/C14H17N3O3/c1-3-17(4-2)10-6-5-9-7-11(13(18)16-15)14(19)20-12(9)8-10/h5-8H,3-4,15H2,1-2H3,(H,16,18)
InChI key
LYBMHAINSFEHRL-UHFFFAOYSA-N
General description
7-(Diethylamino)coumarin-3-carbohydrazide is a coumarin-hydrazide reagent typically used as a fluorescent chemical probe for labeling of cellular carbonyls.
Application
7-(Diethylamino)coumarin-3-carbohydrazide is used for derivatization and detection of carboxylic acid and carbonylated molecules such as lipid peroxidation products (LPP) by electrospray ionization-mass spectrometry (ESI-MS) in positive ion mode. It may be used as a fluorescent label for the determination of lipid aldehydes in food products by normal phase liquid chromatography (NPLC) coupled to ESI-MS and fluorescent detector (FLD) and for the derivatization of monoterpene alcohols in urine prior to analysis by high performance liquid chromatography(HPLC) with FLD coupled to ESI-MS.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Other Notes
Derivatizing agent for carboxylic acid detection
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M.L. Grayeski et al.
Analytical Chemistry, 59, 1203-1203 (1987)
Selective labeling for the identification and semi-quantification of lipid aldehydes in food products
Hollebrands B, et al.
Analytical and Bioanalytical Chemistry, 85(1), 1-9 (2018)
Roland J W Meesters et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 875(2), 444-450 (2008-10-11)
A method was developed for the determination of the monoterpene alcohols verbenol, myrtenol, perillyl alcohol, alpha-terpineol, Delta(3)-carene-10-ol, thymol and p-alpha,alpha-trimethylbenzylalcohol in urine samples. After an enzymatic cleavage of their glucuronide- and sulfate conjugates the monoterpene alcohols were converted in the
Ivana Milic et al.
Analytical chemistry, 85(1), 156-162 (2012-11-29)
Reactive oxygen species (ROS) and other oxidative agents such as free radicals can oxidize polyunsaturated fatty acids (PUFA) as well as PUFA in lipids. The oxidation products can undergo consecutive reactions including oxidative cleavages to yield a chemically diverse group
J C Touchstone et al.
Steroids, 56(12), 601-605 (1991-12-01)
7-Diethylaminocourmarin-3-carbohydrazide was used to label the ketone group of the tetrahydroprogesterones to form fluorescent derivatives with high sensitivity. The four isomeric 3-hydroxypregnanes separated readily on high-performance, thin-layer chromatography after derivatization. This separation was not possible with the underivatized isomers. Standards
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service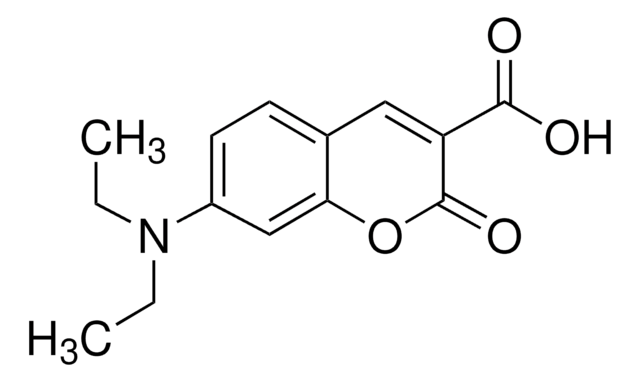
![7-Diethylamino-3-[N-(2-maleimidoethyl)carbamoyl]coumarin suitable for fluorescence, BioReagent, ≥97.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/341/038/a7e0c464-8abe-4eb0-b253-6537f649d89c/640/a7e0c464-8abe-4eb0-b253-6537f649d89c.png)