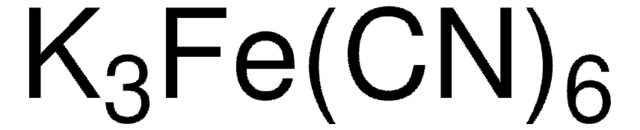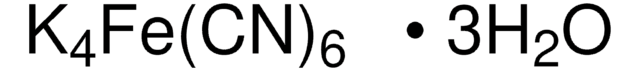P3289
Potassium hexacyanoferrate(II) trihydrate
ACS reagent, 98.5-102.0%
Synonym(s):
Potassium ferrocyanide, Yellow prussiate
About This Item
Recommended Products
grade
ACS reagent
Assay
98.5-102.0%
form
powder
reaction suitability
reagent type: catalyst
core: iron
impurities
≤0.005% Insoluble matter
pH
8-10 (25 °C, 211 g/L)
mp
70 °C (lit.)
anion traces
chloride (Cl-): ≤0.01%
sulfate (SO42-): passes test (limit about 0.01%)
SMILES string
O.O.O.[K+].[K+].[K+].[K+].N#C[Fe-4](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Fe.4K.3H2O/c6*1-2;;;;;;;;/h;;;;;;;;;;;3*1H2/q;;;;;;-4;4*+1;;;
InChI key
NRYOVBQTVIWDNU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Preparation of Carrez I solution.
- Milk sample preparation for gold nanoparticles (AuNPs) based colorimetric detection of melamine.
- Preparation of ferrocyanide/ferricyanide reaction, a redox pair solution for electrochemical testing.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The most frequently used synthetic sweeteners are: saccharin, cyclamate, aspartame, and sucralose (E955), and this application illustrate the analysis of sucralose from soy sauce following the current Chinese national standard method.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service