82800
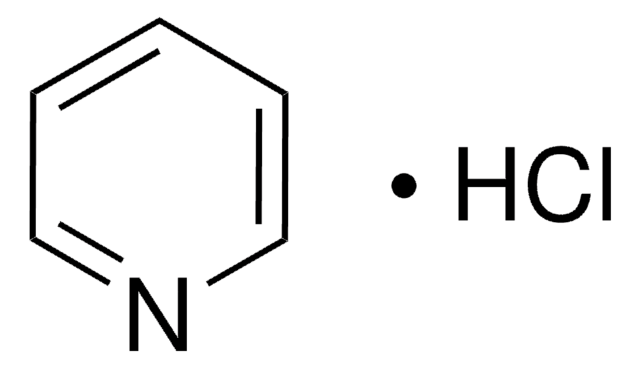
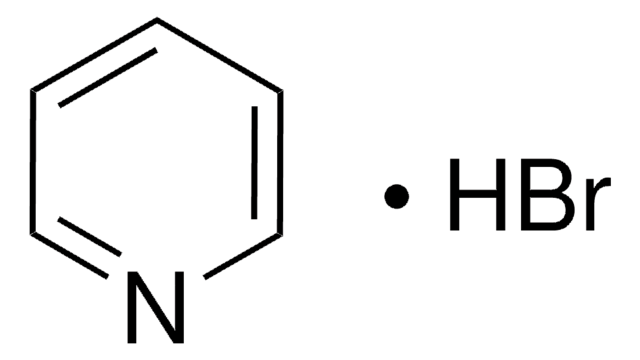
Pyridine hydrochloride
purum, ≥98.0% (AT)
Synonym(s):
Pyridinium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5N · HCl
CAS Number:
Molecular Weight:
115.56
Beilstein:
3615340
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (AT)
form
powder
bp
222-224 °C (lit.)
mp
143-147 °C
145-147 °C (lit.)
SMILES string
Cl[H].c1ccncc1
InChI
1S/C5H5N.ClH/c1-2-4-6-5-3-1;/h1-5H;1H
InChI key
AOJFQRQNPXYVLM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Pyridine hydrochloride can be used:
- As an acid-base catalyst in the conversion of 4-pyridyl propargylic alcohols to the (E)-propenones and propynones.
- As a reagent in the synthesis of 2-arylindene-1-ones , baicalein , pinosylvin derivatives.
- As a reagent in O-demethylation reaction under microwave irradiation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Recent advances in ether dealkylation
Weissman SA and Zewge D
Tetrahedron, 61(33), 7833-7863 (2005)
Estrogen receptor ligands: design and synthesis of new 2-arylindene-1-ones
McDevitt RE, et al.
Bioorganic & Medicinal Chemistry Letters, 15(12), 3137-3142 (2005)
Total synthesis of baicalein
Chen D-Z, et al.
Journal of Asian natural products research, 12(2), 124-128 (2010)
Facile conversion of pyridine propargylic alcohols to enones: stereochemistry of protonation of allenol
Erenler R, et al.
Tetrahedron Letters, 46(34), 5683-5685 (2005)
Synthesis and inhibitory effects of pinosylvin derivatives on prostaglandin E2 production in lipopolysaccharide-induced mouse macrophage cells
Park E-J, et al.
Bioorganic & medicinal chemistry letters, 14(23), 5895-5898 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service