49540
Heptafluorobutyric acid solution
0.5 M in H2O, LiChropur™, suitable for ion pair chromatography
Synonym(s):
HFBA solution, Perfluorobutbutyric acid solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
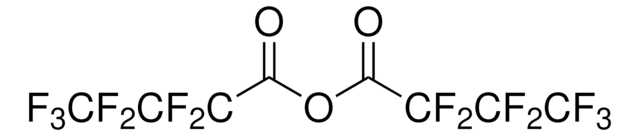
Empirical Formula (Hill Notation):
C4HF7O2
CAS Number:
Molecular Weight:
214.04
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NB.21
Recommended Products
description
concentrate
Quality Level
form
liquid
quality
LiChropur™
concentration
0.5 M in H2O
technique(s)
ion pair chromatography: suitable
SMILES string
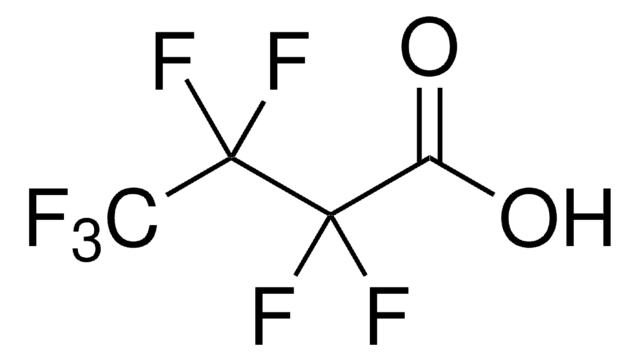
OC(=O)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C4HF7O2/c5-2(6,1(12)13)3(7,8)4(9,10)11/h(H,12,13)
InChI key
YPJUNDFVDDCYIH-UHFFFAOYSA-N
Application
Ion pair reagent for LC/MS
Legal Information
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yuko Murakami et al.
Organic & biomolecular chemistry, 12(48), 9887-9894 (2014-10-31)
Desmosine-CH2, an analog of the elastic tissue degradation biomarker desmosine, can be regarded as a potential internal standard for precise quantification of desmosines by LC-MS/MS. In this study, the chemical synthesis of desmosine-CH2 was completed in 22% overall yield in
Ulrika Eriksson et al.
Environmental science and pollution research international, 20(11), 7940-7948 (2013-04-17)
Diet and drinking water are suggested to be major exposure pathways for perfluoroalkyl substances (PFASs). In this study, food items and water from Faroe Islands sampled in 2011/2012 were analyzed for 11 perfluoroalkyl carboxylic acids (PFCAs) and 4 perfluoroalkane sulfonic
A Hagenaars et al.
Chemosphere, 82(5), 764-772 (2010-11-30)
Perfluorinated compounds (PFCs) are a group of anthropogenic chemicals containing diverse functional groups and chain lengths. They are known to be persistent and bioaccumulative explaining their worldwide environmental presence. The toxicological information on these chemicals is still incomplete and insufficient
Gerold Jerz et al.
Journal of chromatography. A, 1344, 42-50 (2014-04-29)
Betacyanins, red-violet plant pigments, were fractionated by ion-pair high-speed countercurrent chromatography (IP-HSCCC) from leaves extract of Iresine lindenii Van Houtte, an ornamental plant of the family Amaranthaceae. An HSCCC solvent system consisting of TBME-1-BuOH-ACN-H2O (1:3:1:5, v/v/v/v) was applied using ion-pair
Shu-Ching Chang et al.
Toxicological sciences : an official journal of the Society of Toxicology, 104(1), 40-53 (2008-03-21)
Perfluorobutyrate (PFBA) has been detected in precipitation, surface waters, water treatment effluent, and in public and private wells in Minnesota at up to low microg/l concentrations. We evaluated the pharmacokinetics of PFBA in rats, mice, monkeys, and humans to provide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service