Recommended Products
biological source
Escherichia coli K12
Quality Level
form
solution
mol wt
Mr ~220 kDa
packaging
pkg of 1 mL (03707580001)
pkg of 15 mL (03707601001)
pkg of 5 mL (03707598001)
manufacturer/tradename
Roche
storage condition
(Keep container tightly closed in a dry and well-ventilated place.)
parameter
48 °C optimum reaction temp.
technique(s)
activity assay: suitable
color
colorless
optimum pH
6.0-6.5
solubility
water: soluble
suitability
suitable for enzyme test
UniProt accession no.
application(s)
detection
sample preparation
storage temp.
2-8°C
Gene Information
Escherichia coli ... uidA(946149)
Related Categories
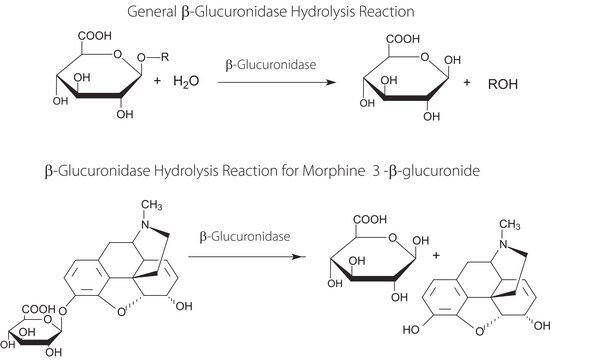
General description
Specificity
Application
- for the hydrolysis of steroid conjugates (glucuronides) in urine (pH 6.0–6.5)
- in doping analysis
- for the detection of benzodiazepine in small doses.
- during sample preparation to cleave off glucuronides prior to GC-MS, HPLC, immunoassays, or other analytical methods.
Biochem/physiol Actions
Features and Benefits
- Perform fast analysis due to the enzyme′s high specific activity.
- Quickly screen for steroids, benzodiazepines, cannabinoids, and opioids.
- Save time by developing your procedure without the need to clean up the reaction or buffer the urine.
Unit Definition
The Fishman unit was previously used. This unit is the release of phenolphthalein from its glucuronide (PPG). It is not possible to measure the relative activities of different preparations for steroid β-glucuronides by comparing their activities with respect to PPG. Many preparation do not catalyze the hydrolysis of PPG, 4NPG, or the various steroid β-glucuronides in urine equally. The choice of 4NPG as standard substrate is based on the following considerations:
- The Michaelis concentrations for the two substrates are similar (KM = 2 ×10-4 M for 4 NPG and KM = 6 ×10-5 M for PPG), but the corresponding rates of hydrolysis differ:
- 4NPG is hydrolysed about 5 × as fast as PPG;
- For PPG, inhibition by excess substrate occurs; this is not observed using 4NPG.
Physical form
(15 ml in one bottle)
Other Notes
For life science research only. Not for use in diagnostic procedures.
Legal Information
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
No data available
Flash Point(C)
No data available
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service