H31859
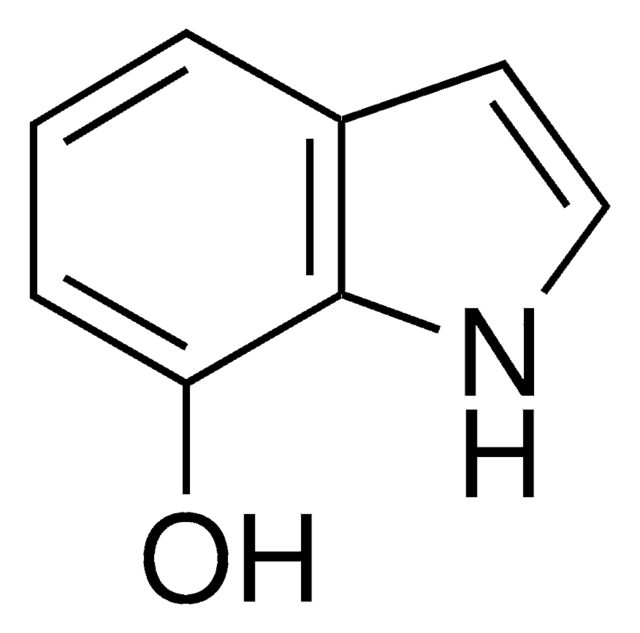
5-Hydroxyindole
97%
Synonym(s):
5-Indolol, 5-Hydroxyindole, NSC 87503
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H7NO
CAS Number:
Molecular Weight:
133.15
Beilstein:
112349
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
106-108 °C (lit.)
SMILES string
Oc1ccc2[nH]ccc2c1
InChI
1S/C8H7NO/c10-7-1-2-8-6(5-7)3-4-9-8/h1-5,9-10H
InChI key
LMIQERWZRIFWNZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Reactant for preparation of (oxoimidazolidinyl/oxopyrimidinyl)benzenesulfonates as antitumor agents and tubulin inhibitors
- Reactant for preparation of anthranilic acids
- Reactant for preparation of indole compounds as dopamine D2 receptor antagonists
- Reactant for preparation of naphthalimide- or carbazole-containing human β-adrenoceptor ligands
- Reactant for preparation of melanins as nature-inspired radioprotectors
- Reactant for preparation of 5-vinyl-3-pyridinecarbonitriles as PKCθ inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiang-Qun Hu et al.
Neuropharmacology, 54(8), 1153-1165 (2008-04-26)
Allosteric modulation of ligand-gated ion channels can play important roles in shaping synaptic transmission. The function of the 5-hydroxytryptamine (serotonin) type 3 (5-HT(3)) receptor, a member of the Cys-loop ligand-gated ion channel superfamily, is modulated by a variety of compounds
Ruth Livingstone et al.
The Journal of chemical physics, 135(19), 194307-194307 (2011-11-25)
Time-resolved photoelectron spectroscopy was used to obtain new information about the dynamics of electronic relaxation in gas-phase indole and 5-hydroxyindole following UV excitation with femtosecond laser pulses centred at 249 nm and 273 nm. Our analysis of the data was
Eva-Maria Karg et al.
Journal of medicinal chemistry, 52(11), 3474-3483 (2009-06-06)
Pharmacological suppression of leukotriene biosynthesis by inhibitors of 5-lipoxygenase (5-LO) is a strategy to intervene with inflammatory and allergic disorders. We recently presented 2-amino-5-hydroxy-1H-indoles as efficient 5-LO inhibitors in cell-based and cell-free assays. Structural optimization led to novel benzo[g]indole-3-carboxylates exemplified
Gretchen Y López-Hernández et al.
Neuropharmacology, 56(4), 821-830 (2009-08-26)
One approach for the identification of therapeutic agents for Alzheimer's disease has focused on the research of alpha7 nAChR-selective agonists such as the partial agonists 3-(4-hydroxy,2-methoxybenzylidene)anabaseine (4OH-GTS-21) and, more recently, 2-[2-(4-bromophenyl)-2-oxoethyl]-1-methyl pyridinium (S 24795). An alternative approach for targeting alpha7
Yu-Bo Wu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(20-21), 1847-1855 (2009-06-02)
To make analytes amenable for fluorescence (FL) detection, polymer monolith microextraction (PMME) coupled to high-performance liquid chromatography with FL detection was developed for the simultaneous determination of catechols and 5-hydroxyindoleamines (5-HIAs) from urine samples. In this method, a two-step pre-column
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service