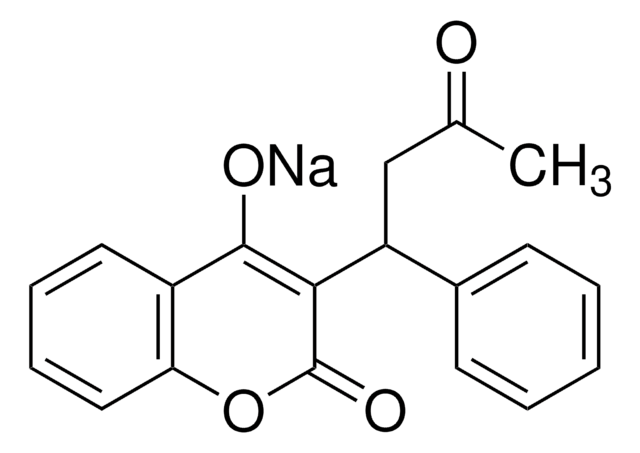
H23805
4-Hydroxycoumarin
98%
Synonym(s):
4-Hydroxy-1-benzopyran-2-one
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H6O3
CAS Number:
Molecular Weight:
162.14
Beilstein:
129768
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
211-213 °C (lit.)
fluorescence
λem 373 nm in methanol
SMILES string
OC1=CC(=O)Oc2ccccc12
InChI
1S/C9H6O3/c10-7-5-9(11)12-8-4-2-1-3-6(7)8/h1-5,10H
InChI key
VXIXUWQIVKSKSA-UHFFFAOYSA-N
Gene Information
mouse ... Maoa(17161)
rat ... Aldh1a2(116676)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karen A Nolan et al.
Journal of medicinal chemistry, 52(22), 7142-7156 (2009-11-03)
The synthesis is reported here of two novel series of inhibitors of human NAD(P)H quinone oxidoreductase-1 (NQO1), an enzyme overexpressed in several types of tumor cell. The first series comprises substituted symmetric dicoumarol analogues; the second series contains hybrid compounds
Ayoob Bazgir et al.
Ultrasonics sonochemistry, 17(2), 447-452 (2009-10-20)
A simple, facile, efficient and three-component procedure for the synthesis of spiro[indoline-3,4'-pyrazolo[3,4-b]pyridine]-2,6'(1'H)-diones by the reaction of 4-hydroxycumarin, isatins and 1H-pyrazol-5-amines in water under ultrasonic irradiation is reported. The advantages of this method are the use of an inexpensive and readily
Mikael I Naumov et al.
The Journal of organic chemistry, 72(9), 3293-3301 (2007-03-29)
2-(methoxymethoxymethyl)aryllead triacetates, obtained in situ from the corresponding arylboronic acids, reacted with 4-hydroxycoumarins, leading to 3-(2-methoxymethoxymethyl)aryl-4-hydroxycoumarin derivatives in good to high yields. These compounds underwent a cascade sequence of reactions, deprotection-halogenation-annulation, to afford polyoxygenated tetracyclic 6H,11H-[2]benzopyrano-[4,3-c] [1]benzopyran-11-ones in good yields.
Katrin J Czogalla et al.
Blood, 122(15), 2743-2750 (2013-08-29)
Since the discovery of warfarin-sensitive vitamin K 2,3-epoxide reductase complex subunit 1 (VKORC1), 26 human VKORC1 (hVKORC1) missense mutations have been associated with oral anticoagulant resistance (OACR). Assessment of warfarin resistance using the "classical" dithiothreitol-driven vitamin K 2,3-epoxide reductase (VKOR)
Benye Liu et al.
Plant molecular biology, 72(1-2), 17-25 (2009-09-17)
Coumarin forms in melilotoside (trans-ortho-coumaric acid glucoside)-containing plant species upon cell damage. In moldy melilotoside-containing plant material, trans-ortho-coumaric acid is converted by fungi to 4-hydroxycoumarin, two molecules of which spontaneously combine with formaldehyde to give dicoumarol. Dicoumarol causes internal bleeding
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service