E50000
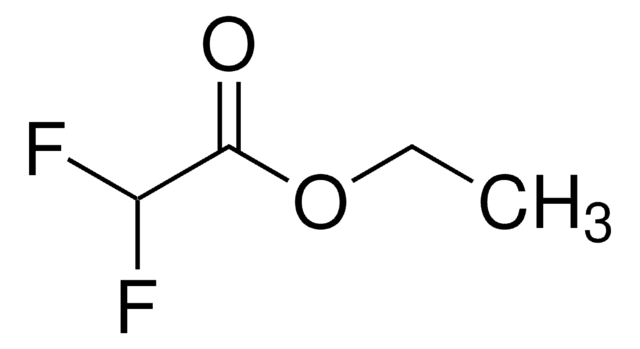
Ethyl trifluoroacetate
99%
Synonym(s):
Trifluoroacetic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
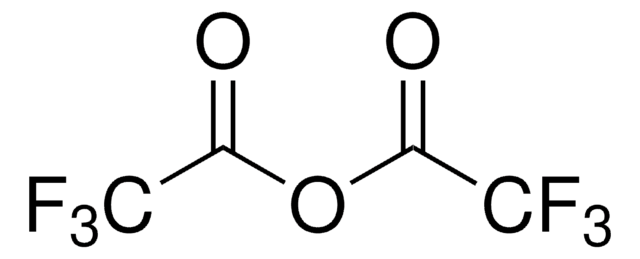
Linear Formula:
CF3COOC2H5
CAS Number:
Molecular Weight:
142.08
Beilstein:
1761411
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.307 (lit.)
bp
60-62 °C (lit.)
density
1.194 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(F)(F)F
InChI
1S/C4H5F3O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
STSCVKRWJPWALQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl trifluoroacetate can be used:
- To synthesize cyclopentenones or furans containing trifluoromethyl group from aromatic ynones.
- In the selective trifluoroacetylation of anilines catalyzed by 4-dimethylaminopyridine.
- As a starting material in the two-step electrosynthesis of trifluoroacetyltrimethylsilane (CF3COSiMe3).
- In the preparation of trifluoromethyl ketones via trifluoroacetic ester/ketone metathesis with alkyl aryl ketones.
- To synthesize o-fluorinated trifluoro acetophenones from substituted fluorobenzene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 4-(Trifluoromethyl) cyclopentenones and 2-(Trifluoromethyl) furans by Reductive Trifluoroacetylation of Ynones
Zhang T and Maekawa H
Organic Letters, 19(24), 6602-6605 (2017)
Highly selective trifluoroacetic ester/ketone metathesis: an efficient approach to trifluoromethyl ketones and esters
Zhou Y, et al.
Tetrahedron, 70(31), 4668-4674 (2014)
Xiaoyan Li et al.
Preparative biochemistry & biotechnology, 47(9), 852-859 (2016-05-26)
Uridine 5'-diphosphate N-acetylglucosamine (UDP-GlcNAc) is a natural UDP-monosaccharide donor for bacterial glycosyltransferases, while uridine 5'-diphosphate N-trifluoacetyl glucosamine (UDP-GlcNTFA) is its synthetic mimic. The chemoenzymatic synthesis of UDP-GlcNAc and UDP-GlcNTFA was attempted by three recombinant enzymes. Recombinant N-acetylhexosamine 1-kinase was used
Non-defluorinative electrochemical silylation of ethyl trifluoroacetate: a practical synthesis of trifluoroacetyltrimethylsilane via its ethyltrimethylsilyl ketal
Bordeau M, et al.
Tetrahedron Letters, 44(19), 3741-3744 (2003)
Synthesis of substituted 3-trifluoromethylbenzo[b]thiophenes
Owton WM
Tetrahedron Letters, 44(38), 7147-7149 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service