A9628
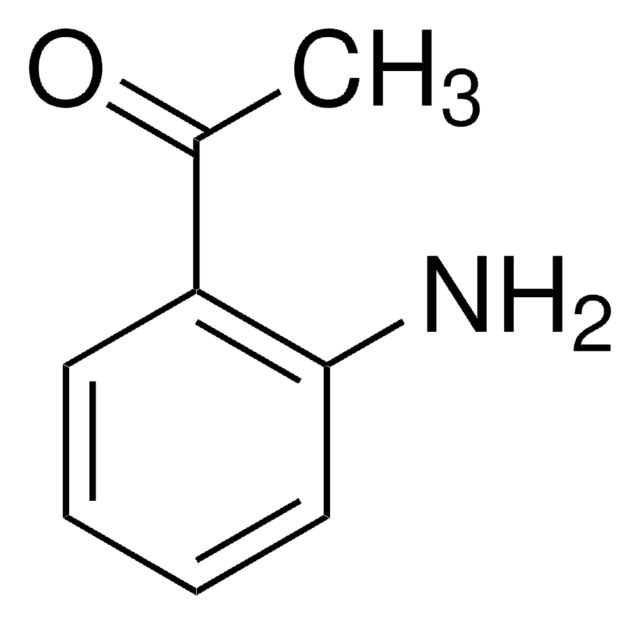
2-Aminobenzaldehyde
≥98%
Synonym(s):
2-Formylaniline, Anthranilaldehyde, o-Aminobenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C7H7NO
CAS Number:
Molecular Weight:
121.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
powder
shipped in
dry ice
storage temp.
−20°C
SMILES string
Nc1ccccc1C=O
InChI
1S/C7H7NO/c8-7-4-2-1-3-6(7)5-9/h1-5H,8H2
InChI key
FXWFZIRWWNPPOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
- Preparation of quinoline derivatives as antiviral agents
- Preparation of electroluminescent materials for OLEDs
- Friedlander-type synthesis
- Preparation of 2-tosylaminophenyl cyclopropylmethanols for gold-catalyzed cyclopropyl carbinol rearrangement
- Benzyl C-H bond amination of arylmethylamines catalyzed by hydroxy-TEMPO
- Silver-catalyzed aniline mediated cascade hydroamination/cycloaddition reactions
Caution
Polymerizes rapidly at room temperature. May yield slightly hazy solution in ethanol due to the presence of a small amount of polymer.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Moamen S Refat et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(1), 234-242 (2007-09-04)
The, nitrin, 2-aminobenzaldehyde phenylhydrazone (2ABPH) was synthesis by refluxing 2-nitrobenzaldehyde with phenylhydrazine in ethanolic solvent. Three transition metal (II) complexes of 2ABPH have been prepared. Elemental analysis, molar conductivity, IR, UV, 1H NMR, and mass spectra, as well as TG/DTG
Yi-Feng Wang et al.
Chemistry, an Asian journal, 4(12), 1834-1838 (2009-10-16)
Generally, amine-catalyzed enantioselective transformations rely on chiral enamine or unsaturated iminium intermediates. Herein, we report a protocol involving dual activation by an aromatic iminium and hydrogen-bonding. An enantioselective aza-Michael-Henry domino reaction of 2-aminobenzaldehydes with nitroolefins has been developed through this
H R Kim et al.
Analytical biochemistry, 223(2), 205-207 (1994-12-01)
We developed an assay system for ornithine aminotransferase (EC 2.6.1.13) using ninhydrin. Pyrroline 5-carboxylate, a product of enzymatic transamination, reacts with ninhydrin under hot acidic conditions to form a reddish pigment soluble in ethanol. The millimolar extinction coefficient of reaction
Riccardo Montioli et al.
The FEBS journal, 286(14), 2787-2798 (2019-04-09)
Among the over 50 gyrate atrophy-causing mutations of ornithine δ-aminotransferase (OAT), the R180T involves an active site residue located at the dimer interface, which in the crystal structure of OAT complexed with 5-fluoromethylornithine engages a salt bridge with the α-carboxylate
Riccardo Montioli et al.
Biochimica et biophysica acta. Molecular basis of disease, 1864(11), 3629-3638 (2018-09-27)
Gyrate atrophy (GA) is a rare recessive disorder characterized by progressive blindness, chorioretinal degeneration and systemic hyperornithinemia. GA is caused by point mutations in the gene encoding ornithine δ-aminotransferase (OAT), a tetrameric pyridoxal 5'-phosphate-dependent enzyme catalysing the transamination of l-ornithine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service